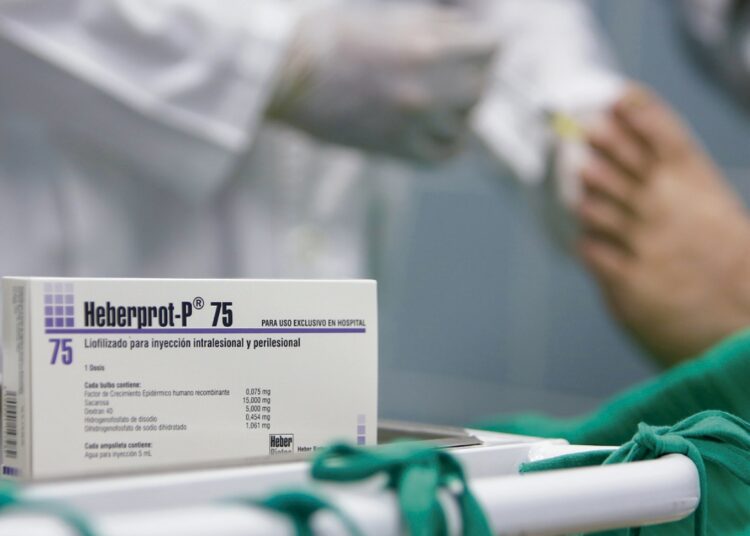
A few days ago, the United States Food and Drug Administration (FDA) authorized the company Discovery Therapeutics Caribe LLC to carry out in the United States a phase 3 clinical trial with the Cuban drug Heberprot-P.
It will be, according to the note, a randomized study. Participants will be randomly distributed into two groups to which they will be administered 75 mg of Heberprot-P or a placebo, that is, a substance that has no therapeutic action. Neither the participants nor the researchers will know what will be administered to each particular patient, which is why it is said that the study will be “double blind.” Once the protocol time has elapsed, the results of both groups will be compared.
This type of study is carried out once the safety and effectiveness of a drug is known, and it is desired to test it in large population groups or introduce it into a market, in this case, the United States.
An MSME in Cleveland, Ohio
According to its website, DTC is a small company based in Cleveland, Ohio. It defines itself as a “late-stage” biotech corporation with “global partners.” It is “committed to pioneering advanced therapies for Americans struggling with diabetic foot ulcer,” according to the same source.
DTC is not going to create any pioneering medicine or innovative therapy to combat this ailment. Its task, which is also legal, is to enable the entry of Heberprot-P into the U.S. market. For this, it has an exclusive license agreement that allows it to market Heberprot-P with the Cuban side, the Center for Genetic Engineering and Biotechnology (CIGB).
To accomplish that, DTC is pursuing what is known as a Biologics License Application (BLA) under provision 351(a) of the Public Health Service Act (PHS Act). This is the traditional way to approve innovative biological products in that country.
The application must contain all the information related to the safety and effectiveness of the drug to be tested, in this case Heberprot-P. That stage has already passed and it is now up to the company to develop a series of studies that demonstrate the effectiveness and safety of the product, before obtaining the license to market it in the United States.
This is possible in accordance with the provisions of Section 515.547 of the Cuban Assets Control Regulation, which authorizes “persons subject to the jurisdiction of the United States” to engage in all types of joint transactions for medical research projects with Cuban nationals and everything related to the marketing, sale and distribution of these products in U.S. territory, of course, complying with a group of strict conditions.
The latter implies the acceptance by the U.S. authorities of the quality of the Cuban biotechnology industry and its products, which is, logically, in the best interest of the citizens of that country.
That does not mean, however, that biotechnological products developed on the island have an open door to that market; it is a small half-open window.
The company has four visible faces: its president and co-founder is Lee C. Weingart, a lawyer from the state of Ohio, who also founded more than twenty years ago LNE Group, a “defense and pressure firm,” what is commonly known as a lobbying firm.
This type of firm is dedicated to defending the interests of groups and companies before government officials, to influence the adoption of public policies, regulations and laws, something perfectly legal and common in the United States.
It is also made up of Jason M. Smith, as co-founder and vice president, who is in charge, among other things, of legal and regulatory matters, as well as relations with the Cuban side.
The medical part of the venture is led by Dr. Milton Sánchez-Parodi, a doctor of Cuban origin who graduated from the Faculty of Medicine of the University of Granada, with more than thirty years of experience in various fields of medicine.
Lastly, Deirdre Kraimer is the director of DTC Clinical Operations. She graduated in chemistry from the University of Connecticut and has a master’s degree in biology from Brown University, both in the United States. Ms. Kraimer, according to the company’s public information, has more than 25 years of experience in clinical research. She will be responsible for designing the clinical trial with Heberprot-P
Diabetes in the world and in the United States
It is very difficult for us not to have an acquaintance or family member who suffers from it. According to the ninth edition of the atlas of the International Diabetes Federation, referring to 2019, we are talking about a serious and chronic disease that appears when the body cannot produce insulin, produces it in an insufficient quantity or cannot use it effectively, for different reasons. The main categories of diabetes are: type 1, type 2, and gestational diabetes mellitus.
The first records of this disease date back to 1500 years BC, Indian doctors used to call it madhumeha, which means “honey urine” and described the differences between the two most important forms of presentation.
In the 2nd century, the pathology that afflicted people who urinated frequently was called “diabetes” in Greece. Diabetes means “siphon” or “pass through…” and the Greeks thought that meat passed through urine and that is why patients became emaciated.
In 1674, British doctor Thomas Willis designated diabetes mellitus as a variant of diabetes in which the urine has a yellowish color, like a “dissolution of honey in a large proportion of water,” adding among its characteristics that it is “more or less sweet to the palate.”
In any case, it is a disease that affects around 463 million people between the ages of 20 and 79, which represents a prevalence of 9.3% among the world population, according to an article published in Pubmed Central. Additionally, this figure is expected to increase to 578 million by 2030 and 700 million by 2045.
According to the WHO, in 2019 1.5 million people died as a direct consequence of the disease, of which 48% were under 70 years old. In addition, another 460,000 people died due to diabetic nephropathy and it is estimated that hyperglycemia, one of the signs of the disease, caused around 20% of deaths of cardiovascular origin. In all fairness we can say it is a pandemic.
In the United States, the market that Heberprot-P aims to conquer, there are 38.4 million diabetic people, that is, 11.6% of the population of that country, which also has 8.7 million still waiting for a diagnosis, according to a report from the Center for Disease Control and Prevention (CDC).
Additionally, 97.6 million people over 18 years of age in that country are considered prediabetic and the trend is for these numbers to continue increasing.
What is diabetic foot and what is its impact on global health?
Diabetes, as is known, can cause damage to multiple organs and systems including the heart, kidneys, eyes, nerves and vascular system.
A frequent and fearful complication of the disease is the diabetic foot ulcer (DFU). It affects 6.3% of patients, according to an article that appeared in the World Journal of Diabetes in December 2022. DFU is understood as symptoms that include “cracking, ulceration, infection or destruction of foot tissue” in diabetic patients, according to that source.
It is a multifactorial phenomenon that in the Western world is the cause of more than 60% of non-traumatic amputations. Furthermore, it is responsible for a considerable increase in hospitalization and mortality rates in these patients and for a significant reduction in the quality of life of these people.
Standard treatment of the disease includes pressure relief from the injured area (basically not supporting the injured limb); applying saline solution and dressing to provide a moist environment for the area; debridement, which involves the removal of dead or damaged tissue to promote healing, also done when necessary.
It also includes treatment with antibiotics and surgery, in case of infection. This is combined with strict glycemic control and the correction of peripheral vascular insufficiency, that is, the disease characterized by decreased blood flow in the extremities and which is common in diabetic patients.
Among the innovative treatments for ulcers are human cell cultures, the application of hyperbaric oxygen, that is, at high pressures, and the use of recombinant epidermal growth factor (R-EGF), which is the active ingredient of Heberprot-P.
In this context and given the high incidence of the disease in the world, it is obvious that there is great interest in the drug produced on the island. Heberprot-P reduces the incidence of amputations and promotes healing, which is the golden goal in this type of patient.
One of Cuban Biotechnology’s star drugs
The history of Heberprot-P dates back to the 1960s, when the future winner of the Nobel Prize in Medicine and Physiology in 1986, Stanley Cohen, isolated a protein capable of stimulating cell growth in the submandibular glands of a male mouse. He called it Epidermal Growth Factor (EGF).
During the 1970s, EGF was found in various human body fluids and, a decade later, specifically in 1988, Cuba became one of the first countries in the world to obtain it artificially by recombinant methods, which added the initial R to the original acronym: R-EGF. This made it possible to develop a series of investigations in which this molecule was the protagonist.
“The father of Heberprot-P,” Dr. Jorge Berlanga Acosta, in 1994 was researching nerve regeneration. However, upon reading the work of a British group he became excited, because they had shown that injecting EGF could prevent cell death.
At first it was thought that superficial application of EGF would be enough for it to take effect. What the British discovered and that motivated the Cuban scientist was the need to inject it specifically so that the desired results could be obtained.
That moment can be considered the starting point of Heberprot-P. Of course, nothing is the result of chance, but rather the talent and hard work of the researcher and his team, who for seven years carried out a series of investigations in rats and pigs with very encouraging results in the field of healing. These basically consisted of causing wounds in the animals and then administering the medicine to study its effect.
Dr. Jorge Berlanga Acosta. Photo: Taken from Cubaperiodistas (online).
In the early 2000s, Dr. Berlanga and his team made the leap into human research. In this way, EGF was tested in different pathologies, such as hemorrhagic cystitis after bone marrow transplants and also in patients with colitis.
However, the enormous potential of this molecule in diabetic patients must have become evident, because since 2006 research began to be published in which the beneficial effect of intralesional infiltration was demonstrated, that is, injecting EGF into the DFU, to prevent amputation in 6 out of 10 patients with the diabetic foot ailment, particularly in seriously ill patients.
Since Heberprot-P obtained its first health registration in 2006, the license to be used in Cuba, to date, it has been approved in 26 other countries, where it has been applied to more than 390,000 people, according to an article that appeared in the Cuban Journal of Public Health.
It is very significant that such a novel product, unique in its kind and proven to be efficient, has not reached the U.S. market until now, where diabetes has a high prevalence.
Heberprot-P in the United States, a difficult story
Evidently, its absence in that market has not been the result of ignorance of the drug’s potential. Heberprot-P has been known in that country for more than a decade. In fact, Congressman Joe García, who advocated for the marketing of this product in his country, was harshly criticized by politicians and right-wing media.
On the other hand, it is not the first time that a U.S. company has tried to go through the tangled jungle of prohibitions of the Cuban Asset Control Regulation (CACR) with the aim of marketing Heberprot in that country.
Since 2015, a company called Mercurio Biotec began taking steps to this end, which included numerous meetings in Washington D.C. and in Havana. In 2018, these negotiations reached what seemed to be a good conclusion, materialized in an agreement with the Cuban company Heber Biotec S.A. This was considered historic in the context of the very flawed relations between the two countries.
That agreement was widely reported by the press, but things did not reach a happy ending.
It is important to remember that, after the timid thaw at the end of the Obama administration, relations between the two countries fell to their lowest point in decades, and then the pandemic hit. The agreement between Heber Biotec S.A. and Mercurio Biotec was one of the victims of these circumstances.
It is curious that the then president of Mercurio Biotec was Dr. Milton Sánchez-Parodi, who is now responsible for the medical part of the recently announced collaboration between DTC LLC and the CIGB. Dr. Sánchez-Parodi continues efforts to bring Heberprot-P to the U.S. market.
It is also valid to note that the approval of clinical trials by the United States regulatory agency, the FDA, does not mean that restarting this effort has been attempted only now. Since the first quarter of this year the company had requested authorization.
According to a report from Prensa Latina news agency, the study should last some 18 months and would include around “180 patients in about 25 centers” in the country. Additionally, the Cuban product is expected to be on the market “in mid-2028,” according to that source.
This would mean relief for the 160,000 Americans who are amputated every year for this reason, of which 80,000 die in the five years following the amputation, making DFU one of the leading causes of death in the United States, even above some types of cancer.
The success of the collaboration that many of us await would be a milestone in relations between the two countries and renewed hope for many patients.