In mid-2020 we heard the name Jusvinza for the first time. A state of emergency had just been declared due to the COVID-19 pandemic in Cuba, but it was already evident that any help we had to combat the virus would be useful. This is how we began using Jusvinza, a drug originally designed to combat rheumatoid arthritis, in the country’s intensive care rooms. We saw with satisfaction that it was an effective medication not only in seriously ill COVID-19 patients but in many other critical cases in which systemic inflammatory response syndrome (SRIS) was involved.
Today we know how many Cubans have died from the fearsome virus. However, we have no idea how many lives this and other drugs helped save, the result of decades of work by prestigious and established Cuban researchers. Faced with the health crisis, Jusvinza and other medications became part of the Cuban protocol for the treatment of seriously ill patients; that is, they were in the last line of combat against COVID-19, in those with the greatest propensity to die.
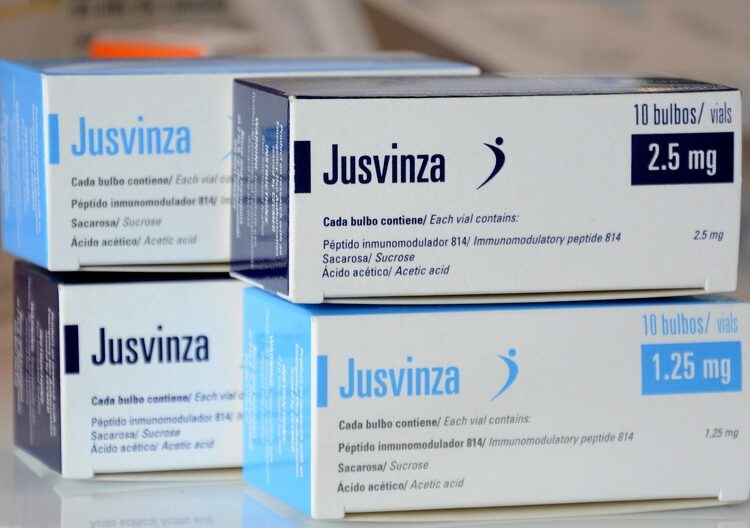
Life continued its course and, since September of last year, Jusvinza was approved by CECMED, the Cuban regulatory agency for Medicines, Equipment and Medical Devices, for the purpose for which it was created: the treatment of rheumatoid arthritis.
In fact, since October 2023, the new drug that could change the lives of tens of thousands of Cubans and millions of people around the world who live with the disease had entered the final phase of clinical trials.
What is rheumatoid arthritis? What are its causes? Why is Jusvinza a ray of hope for Cuban patients? How was it possible to develop an immunomodulatory drug like Jusvinza?
Rheumatoid arthritis (RA), the most common autoimmune disease in the world
According to the corresponding chapter of the MSN Manual, RA is “a chronic systemic autoimmune disease that affects the joints.” The pathology damages the entire body, which is why it is systemic; there is no cure, hence it is chronic, and it is due to disorders in the immune or defensive system, which attacks the body itself and harms it, something like a “friendly fire.”
According to the WHO, in 2019 there were 18 million people affected by the disease in the world, of which 7 out of 10 were women, causing moderate or severe symptoms in 13 million. It is a pathology that usually appears after the fourth decade of life; in fact, 55% of those affected that year were over 55 years old.
RA usually begins with general and nonspecific symptoms such as fatigue, weakness, and occasionally a low fever. This is accompanied by pain, stiffness, and swelling in the joints. The disease then progresses rapidly, with 80% of patients developing some type of permanent joint abnormality within the first ten years.
A characteristic of RA is that the classic symptoms of inflammation (pain, heat, redness, increase in volume, and functional impotence, that is, the inability for the normal functioning of the affected structures) are symmetrical, which means that it attacks similarly on both sides of the body and affecting the small joints of the hands and feet. In addition, it can damage other major ones, such as the shoulder, hip, elbow, and spine. A typical symptom is stiffness, which can last more than an hour after the patient gets up or after long periods of physical inactivity.
Extra-articular manifestations of the disease include rheumatoid nodules, which can be found under the skin and in other locations such as the lungs. There is also inflammation of the blood vessels, known as vasculitis, neuritis, when it attacks the nerves, or myocarditis and pneumonitis, if it affects structures of the heart and lungs, respectively.
The treatment for RA includes a range of general measures or treatments such as rest, physical exercises, proper diet, splints, and surgery. However, in the case of the disease, the greatest weight is given to pharmacological treatment, whose fundamental objective is “to reduce inflammation to prevent erosions, progressive deformity and loss of joint function.” The dream goal with this approach is the total disappearance of symptoms or minimizing the activity of the factors that cause the disease.
The first line of treatment is so-called DMARDs or disease-modifying drugs. Among them, the paradigmatic example is the MTX. According to the Clinical Rheumatology site, this medication “partially inhibits the immune system” and reduces “long-term autoimmune joint inflammation,” through mechanisms that are not entirely clear.
Despite its effectiveness, one of the drawbacks is the adverse reactions it produces, which can include lung and liver toxicity (which in some cases leads to liver failure) and a decrease in platelets and the body’s defensive cells.
Then we would have biological agents, basically any pharmacological substance manufactured in the laboratory from a living organism. These include vaccines, immunotherapy, stem cell therapy, and gene,1 among others.
In the case of RA, drugs inhibit or block different mediators of the inflammation process, such as tumor necrosis factor (TNF)-alpha and some lymphocytes, defensive cells, which are present in the genesis of this disease. The biggest concern that these medications generate is that they predispose to the appearance of infections, which can sometimes be serious, as well as neoplastic diseases.
There are also steroidal anti-inflammatory drugs (prednisone) and non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen. The former tend to be used at low doses and, if possible, avoided, due to their numerous side effects. NSAIDs, for their part, play an important role in relieving symptoms, but they do not stop the progression of the disease.
Inflammation and autoimmune diseases
We will briefly explain what inflammation is, to understand the mechanism of operation of Jusvinza, the Cuban medicine against RA, and why it is so novel.
Let’s start because the classic signs of inflammation were described by Celsus two thousand years ago. Eighteen centuries later, the Scottish surgeon John Hunter reached a revolutionary conclusion that remains completely valid: “Inflammation is not a disease, but a nonspecific response that produces a healthy effect in the body.”
Indeed, when damage of any type occurs, the body reacts intending to isolate and repair the affected area in the shortest possible time. It occurs through a very complex mechanism that includes dozens of substances that we will call, generically, inflammatory mediators.
They are responsible for warning the immune system that something is happening and guiding it to the affected area, so that the amount of blood that reaches there increases, which is the basis of redness and increased temperature, signs of the beginning of the inflammatory process. Likewise, antibodies are released, specific proteins that the body uses to attack enemy agents (viruses, bacteria, toxins…), and cells of the defensive system with specific functions are recruited.
After a while, a mechanism operates to regulate the inflammatory process. This is essential, because when the inflammatory response gets out of control, that is, it cannot be mitigated, the so-called systemic inflammatory response that we talked about at the beginning of the article appears, known as SIRS. This is considered a syndrome and has a high mortality rate; in fact, it is the main cause of the majority of deaths from the COVID-19 virus.
Furthermore, the organism must be able to recognize the difference between what is its own and what is foreign, to avoid “friendly fire,” which is nothing more than the process of attacking itself. When the identification system fails, diseases known generically as autoimmune appear, in which, for different reasons not fully explained, this tight clockwork mechanism that is the defensive system does not function properly. Within this group, we have pathologies such as allergies, asthma, lupus, and RA, as part of a very long list.
History of a Cuban drug
What happened during the COVID-19 pandemic with the international race whose goal was vaccines resulted in the concept going from the concept to the application of immunizers to millions of people in just ten months. It is something unusual that is unlikely to happen again: it normally takes much longer for any pharmacological product to demonstrate, before being available for use, that it is effective, safe, and of sufficient quality to be administered to people.
It is naive to think that coincidences occur in science. In fact, the most famous of these is the discovery of penicillin by Sir. Alexander Fleming has been questioned more than once. Rather than having found it randomly, the famous English bacteriologist found it after more than a decade of searching for an antibiotic.
In the case of Jusvinza, it is also a work of more than two decades, led by Dr. C María del Carmen Domínguez Horta, a Cuban researcher, graduate in biochemistry from the University of Havana, doctor of sciences and professor of the Latin American School of Medical Sciences.

Jusvinza is based on research that, according to the MSD website, is “a systematic and refined thinking technique that uses special tools, instruments, and procedures to obtain the most appropriate solution to a problem.” This is an expensive and long process, which consists of four phases and usually takes between 10 and 15 years; in the case at hand, the COVID-19 pandemic has delayed it even more.
The first stage is discovery, in which a medical need for a specific disease is identified. In this case, to provide new drugs for the treatment of RA, because those available on the market are either not completely effective or generate too many adverse reactions and side effects that limit their safety.
Since 2006, Dr. Domínguez Horta has been publishing research on this topic, emphasizing Cuban patients to determine their specificities.
In 2009, the researcher and her team already had a compound at that time called ALI1 or CIGB814. It is a peptide, in very simple words; a fragment of the HSP60 protein. This is part of a group of proteins present in numerous organisms, from single-celled organisms to humans, and that fulfill many functions within cells. Since the beginning of the century, there was evidence that this family of proteins could regulate inflammatory processes that cause RA.
To obtain the product, bioinformatic techniques were used that allow “research, development, and application of computer and computational tools to allow and improve the management of biological data.” In this way, CIGB814 was obtained, which began to be tested in laboratory rats, in which it was shown that it decreased the blood concentration of an important mediator of inflammation called tumor necrosis factor-alpha (TNF-alpha) and that it increased the production of regulatory cells of the immune system. This decreased joint damage in the laboratory animals, demonstrating the effectiveness of the drug.
In research and clinical trials carried out during the decade of 2010-2020, it was shown that the drug was effective in reducing inflammation through different mechanisms in patients in the active phase of RA. This guaranteed its viability and it began to be tested in clinical trials to investigate its safety, which was also demonstrated in small groups of patients during this phase.
The phase II studies were interrupted by COVID-19 and here the history of the drug takes an interesting turn.
Dr. C Domínguez Horta and her team understood that SARV-CoV-2, the virus responsible for the pandemic crisis, acted through the same inflammatory mechanisms in severe and critical patients, and they saw the possibility that the drug, today commercially named Jusvinza, be used to treat these patients, at a time when no specific treatment existed.
For this, the drug had to be endorsed by the Cuban regulatory agency, CEDMED, after several trials in patients in which it was shown to be effective and safe.
Jusvinza is passing the last stage of the clinical research phase. We will have to wait to see what results are obtained once the vaccine is tested on thousands of people.
A path that has just begun
RA is a disabling disease that affects the quality of life of millions of people who suffer from it and in some cases can cause serious damage to health. Furthermore, although there are effective medications for its treatment, they do not have the best safety profile. Many of these drugs, on the other hand, are not available to Cuban patients because they are produced by U.S. pharmaceutical companies or because of their high costs.
To give you an idea, according to a 2013 study, the average annual treatment cost for a patient with RA using three biological drugs (not in unison) similar to Jusvinza was 11,957.96 euros in Spain, reaching, in the case of Adalimumab, one of these, at 13,073.19 euros. Keep in mind that 1.2% of the Cuban population suffers from this disease, that is, we are talking about tens of thousands of people.
The long road that the creators of Jusvinza have had to travel, especially the project leader, Dr. C María del Carmen Domínguez Horta, is an example of how complex obtaining medicine usually is. Its use in critically ill patients during the COVID-19 pandemic and in other seriously ill patients with SIRS also demonstrates that, sometimes, unfortunate events such as the pandemic open new opportunities for science.
Jusvinza’s journey has not ended, rather, it is now just beginning. With its approval by CECMED, the possibility of using the drug to treat the local population that needs it opens up, as part of the open study from which more than 66,000 people will benefit, according to Biocubafarma. However, for its commercialization in other markets, it is necessary to move beyond Phase III of clinical trials, which it is now in.
Everything seems to indicate that there will be a ray of hope in the lives of patients who currently rely on conventional treatment.
________________________________________
Note:
1 Sometimes a single defective gene can be the cause of diseases that damage the quality of life of those who carry them and can even cause their death. Through gene or genetic therapy, genes that cause major health disorders can be replaced, others that help fight diseases can be added, or some that cause health problems can be inactivated. We have an exciting example of these techniques with the new treatments for sickle cell disease, to which we dedicate an article in this section.







Can Jusvinza by good,help to hills the Ankylosing spondylitis? Routine MRI of the thoracic and lumbosacral spine was performed. Mild movement artifact. Mild kyphosis of the thoracic spine noted. Thanks.
Jusvinza has been shown to be effective and safe in the treatment of the inflammatory response, so it could be useful in Ankylosing spondylitis. However, studies must be developed to demonstrate this before being administered to patients.
Hello
İ have got that disease named romataid artrit.i live at Türkey and i want to talk about your healing programme.pls write me
Anyone has an idea when it will be in the market?
The drug is currently being studied. I hope it can be on the market soon.
By March 2025 , clinical trials’ outcome will be available for public.
Does the drug alter or stops the progression of RA
My wife has it and lives in Cuba and I believe that it was going to be administered
I hope
Your team has done a great phenomenal work
Thank you
Anthony Trujillo