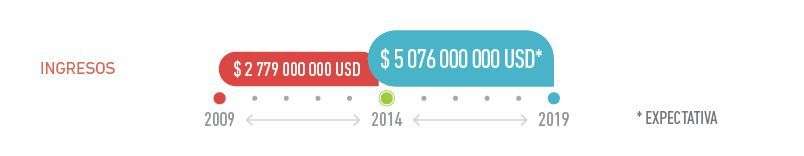
BioCubaFarma aims at exporting about 5 076 million dollars in the next five years. This Group of Biotechnological and Pharmaceutical Industries was founded in November 2012 and is in charge of the processing of materials by biological agents for producing medicines, equipment and high technological services for improving the population’s health and the generation of exportable goods and services in the current Cuban context.
The Group is made up of 38 large companies comprising 21 613 workers. It emerged from the fusion of Quimefa, an enterprise for the production of medicines, and the Biotechnology Scientific Pole, leading institutions in this field in Cuba.
According to Dr. Agustin Lage, general director of the Molecular Immunology Center, BioCubaFarma’s main challenges are:
– To grow, rely on permanent investment capacities, reach international quality standards (which are not static), diversify exports with a commercial strategy up to par with this purpose, guarantee the constant renovation of products.
Disadvantages
– Scarce resources, industrial underdevelopment, economic blockade, high migration rates of qualified personnel, deteriorated work and life conditions, objective limitations in the use of TICs and connectivity and internet access.
In view of this context, the development strategy by the Cuban biotechnological sector can be summarized as follows:
– Strong governmental investments, Support in Cuban scientists and professionals, Closed cycle strategy: from research to post-commercialization monitoring, National cooperation: coordination among research and development institutions and those implementing results, Integration of institutions:
Research+Development+Production+Commercialization, View of Biotechnology as part of the Cuban Health System, National market as priority, Interest in gaining in international competitiveness: quality, production volumes, costs, news, joint ventures; High investments in education and training of human resources.
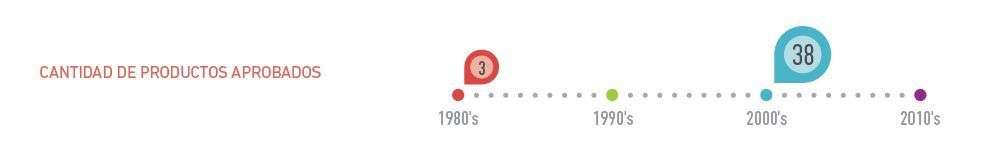
The implementation of this formula has represented an exponential increase of production rates in a couple decades. In 2011, Cuba had most extensive and intense vaccination program (13 vaccines). The Scientific Pole in the West of the Capital, founded in the early 80’s, owns more than 900 patents abroad and some of them have been recognized with a Medal by the Copyright World Organization.
The Cuban industry in this field is spread throughout 58 nations in the world and has created joint ventures with some of them.
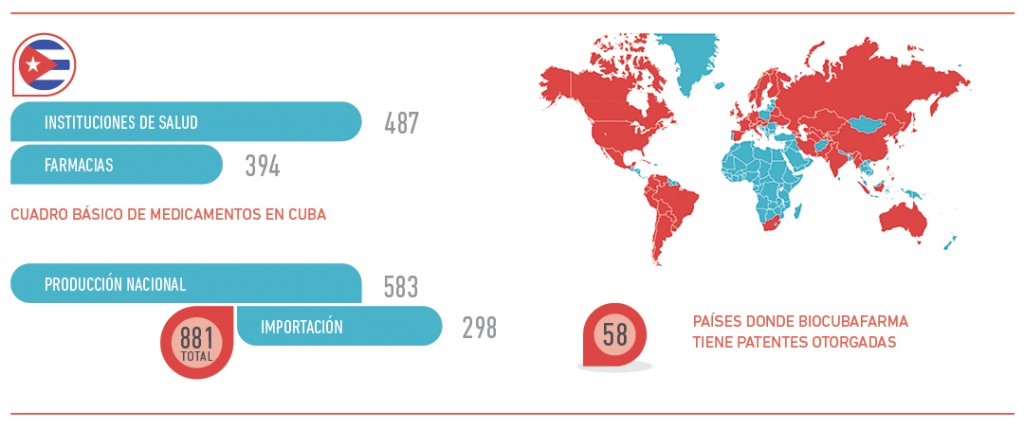
Even though the national market is top priority, there are difficulties in terms of:
Deficiencies in:
– The distribution of medicines for supplying the population and in its organization and legal aspects
Failures in:
-Imports, investments, quality of the services
Some of the achievements of Cuban biotechnology are:
– First therapeutic vaccine for the treatment of advanced lung cancer(CIMAVAX EGF); also useful in head and neck, brain, gastric, breast, rectal, prostate, neck of the womb, bladder, ovary and pancreas cancer.
– Vaccines against hepatitis B; antimeningococcica type B (VA-MENGOC-BC), unique in its kind in the world; against tetanus, leptospirosis and typhoid fever; against meningitis B and C, and a therapeutic vaccine against all varieties of dengue.
– Immunization against Haemophilusinfluenzae, the first synthetic vaccine in the world.
– Interferon against cirrhosis of the liver and liver cancer.
– Heberprot-P, the single medicine in the world which speeds up the healing of diabetic foot ulcers and reduces the risk of amputation of the limbs.
– Substances that can fight malignant tumors: Vidatox, made from scorpion venom, and CimaVax, the first therapeutic vaccine against lung cancer.
– More than one hundred monoclonal antibodies.
– Diagnosis systems for diseases such as HIV-AIDS and lipoprotein (a).
– Skin growth factor.
– Medical equipment and computer programs.
Policies for the negotiation of projects by the Center of Genetic Engineering and Biotechnology (CIGB by its acronym in Spanish):
– Each project will be subjected to independent negotiation.
– After apotential counterpart expresses its intention of starting negotiations, a confidentiality agreement will be signed (if required) prior to the exchange of additional sensitive information on the subject of work.
– A negotiation team, prepared for discussing thoroughly the state of technology or research, status of patents, financial requirements, competence status, market estimations, work schedule and risks will be appointed for each project.
– In general, the agreement must lead the counterparts to share the project’s development costs in accordance with a pre-established payment structure.
– The Cuban counterpart tangible assets or productionfacilities, pilot or research, belonging to the Cuban State are ruled out of all kinds of negotiations.
– Out of this exception, the rest of the negotiation terms will be analyzed and agreed in a flexible and mutual understanding environment.
Projects in progress:
– Therapeutic vaccine against hepatitis B, Vaccine against hepatitis C, Recombining vaccine against dengue, AIDS vaccine, Therapeutic vaccine against cervix cancer, Cancer immunotherapy, Identification of cell targets of inhibition of HIV, Autoimmunity, Design of antiviral molecules against HIV.
The Cuban biotechnological industry demands competition in first world markets. They are working on the development of several vaccines and on different projects for others with commercial purposes and are –relatively– close to materializing.










