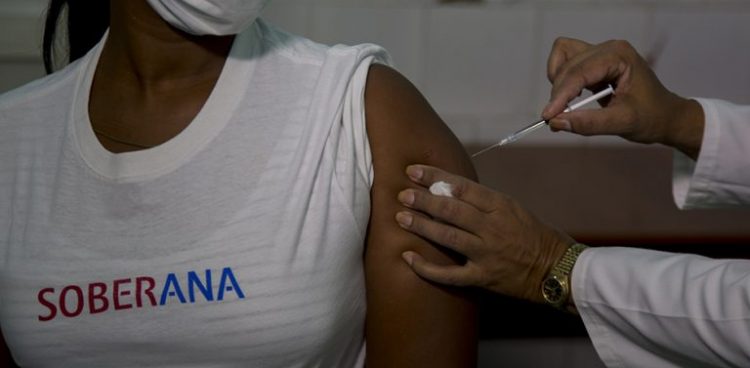
This Monday Cuba began in Havana the clinical trials in humans of its first vaccine project against the SARS-CoV-2 coronavirus, the first two phases of which will last until the beginning of November and will involve 676 volunteers.
The trials of the formula, developed by scientists from the state-run Finlay Vaccine Institute and called Soberana 01, were authorized after its registration on August 13 in the registry of the Center for State Control of Medicines, Equipment and Medical Devices (CEDMEC).
The Cuban vaccine successfully passed the animal experimentation stage between May and August and is the first vaccine candidate from a Latin American country approved for clinical trials, according to the director of the Finlay Institute, Vicente Vérez.
In the initial stage, the vaccine will be administered at the National Toxicology Center (CENATOX) to 20 volunteers between 19 and 59 years old and a week later to another 20 people between 60 and 80 years old, those responsible for administering the vaccine explained.
Dr. Carlos Alberto González, head of the CENATOX Clinical Trials Unit, explained to Cubadebate that, when chosen as the clinical trials unit, his institution is “responsible for ensuring good clinical practices, in such a way that the subject’s safety is well protected and that the data are reliable, traceable, reproducible so that this study is transparent and auditable by the competent authorities.”
The second phase will begin on September 11 with the vaccination of the remaining volunteers until reaching the planned 676. Participants in the trials will receive two doses of the vaccine 28 days apart and their response will be studied over two months.
The Finlay Institute has set January 11, 2021 for the completion date of the vaccine study, the results of which would be available on February 1 to be published on the 15th of the same month.
Vérez explained when presenting the research that the clinical trial will be “randomized, controlled, adaptive and multicenter” and has the objective of evaluating the “safety, reactogenicity and immunogenicity” in the two-dose scheme.
Although human trials are beginning now, on July 28, three of the researchers from the team that developed the vaccine candidate inoculated themselves with the formula.
Cuba: COVID-19 vaccine project already tested on three researchers
Compared to the other most advanced vaccine projects today, developed from adenoviral vectors or inactivated viruses, the Cuban formula is based on a recombinant protein, although at the moment no further data on its development has been officially released.
The island, which has to date 3,717 cases of coronavirus―545 active cases―and 91 deaths from COVID-19, has faced outbreaks of the disease in its western provinces in recent weeks. The most complex situation is in Havana, where the authorities have reactivated the restrictive measures that had been relaxed in early July.
Cuba has a renowned biotechnology and pharmaceutical industry that currently produces eight vaccines against diseases such as meningitis, lung cancer (therapeutic) and solid tumors, among others.
EFE/OnCuba