Last July 17, a group of people devoted to science in Cuba finally had in their hands a small bottle with a transparent liquid; on the label it said: COVID-19 vaccine. What is in that precious small amount of liquid, how to get there and what time is needed to have a Cuban COVID-19 vaccine?
What is a vaccine?
According to the World Health Organization (WHO) “a vaccine is understood to be any preparation intended to generate immunity against a disease by stimulating the production of antibodies. This may be, for example, a suspension of killed or attenuated microorganisms, or products or derivatives of microorganisms. Most vaccines are given by an injection, but some are given orally (by mouth) or sprayed into the nose.”
What does it mean to generate immunity by stimulating the production of antibodies?
The immune system is like the body’s defense army, and it is a complex army where different body organs and cells participate. From the moment we are born we have the ability to defend ourselves against external agents that can pose a threat to health, when these are biological entities capable of causing diseases they are called pathogens. There are many pathogenic microorganisms for humans (bacteria, fungi, viruses, parasites). When we come into contact with them, our body has certain defense systems prepared, and this pathogen has to overcome physical and biological barriers to enter our body, multiply and cause disease, this first line of defense is called innate immunity.
However, the proof that this innate immunity is not enough is that we get sick, that we catch colds, or get diarrhea of viral or bacterial origin, or chickenpox, or tuberculosis, or hepatitis, or lesions on the skin caused by fungi, or HIV, or herpes, among many other infectious diseases. That’s because that rapid first response is not specific to each type of pathogen and is not always powerful enough to protect us. So our body has designed another line of defense: acquired immunity. Many times we hear our grandparents say: let the girl walk barefoot, she has to get antibodies—almost always the accumulated wisdom has some reason. I would like to say that acquired immunity, which is a slower, but more specific and efficient response, is not only about antibodies, but these play a very important role in protecting against infectious pathogens.
What are antibodies?
One of the most powerful weapons of the immune system is antibodies (also known as immunoglobulins). Antibodies are molecules (glycoproteins) produced by a type of cells of the immune system (T lymphocytes). When our body comes into contact with a pathogen, T lymphocytes are activated and produce antibodies against that specific type of pathogen.
Antibodies have two main functions: they mark these pathogens to be attacked and killed by other cells of the immune system, or they are bound by a specific part of the pathogen that blocks their ability to enter and harm cells in the body.
Antibodies (which are such specific molecules) are not generated against the entire pathogen, but against parts of them, that substance (that part of the pathogen) that generates the production of antibodies is called antigen.
Pathogenic microorganisms are complex structures. Determining within them which antigen is going to generate an immune response powerful enough to protect us from disease is one of the most important steps in the design of a vaccine of this type.
The objective of a vaccine is to put us in contact with the pathogen (or with its specific antigens) in a safe way―without causing the disease―so that our immune system produces these specific antibodies, because once produced our immune system will keep that “memory” and if we come into contact with the live or active pathogen, all these “specific weapons” will be ready, the response will be faster, most of the time it will protect us, and if we contract the disease it is usually much less serious.
COVID-19
The current pandemic is caused by a virus. Within microorganisms, viruses are very particular entities. While bacteria and fungi can live and reproduce in various environments: land, air, water, rocks, minerals…viruses are obligated intracellular parasites, that is, they cannot reproduce outside a cell.
A virus is a small collection of genetic code, either DNA or RNA, surrounded by a protein coat. A virus cannot replicate alone. Viruses must infect cells and use components of the host cell to make copies of themselves. Often, they kill the host cell in the process, and cause damage to the host organism. Viruses have been found everywhere on Earth. Researchers estimate that viruses outnumber bacteria by 10 to 1. Because viruses don’t have the same components as bacteria, they cannot be killed by antibiotics; only antiviral medications or vaccines can eliminate or reduce the severity of viral diseases, including AIDS, COVID-19, measles and smallpox
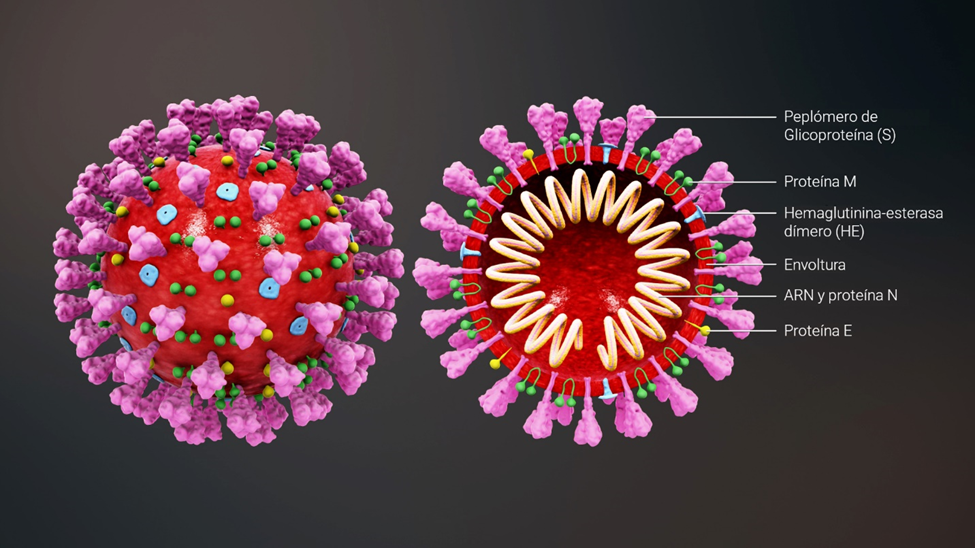
Structure of the SARS-CoV-2 coronavirus
SARS-CoV-2 is an enveloped virus with a positive RNA strand. The “key” to enter the cell is found in the so-called “spike” proteins (S), which cover the virus envelope.
*Caption:
S spike (peplomer) glycoprotein
M protein
Hemagglutinin esterase (HE)
Envelope
RNA and N protein
E protein
What does the Cuban Soberana-01 vaccine candidate consist of?
There are different types of vaccines, from those that use the live attenuated pathogen, vaccines that use the killed (inactivated) pathogen, vaccines with toxoids, vaccines that are based on genetic technology (DNA or RNA), to many types of subunit vaccines (they use a part of the pathogenic agent―antigen―with a proven immunogenic capacity, that is, capable of inducing the immune response). The Cuban SOBERANA-01 vaccine candidate is in this last group.
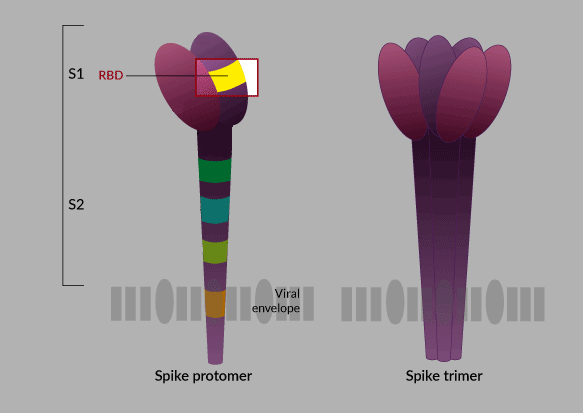
It is logical to imagine that the success of a subunit vaccine depends to a large extent on the antigen that is chosen to produce it. In the case of the researchers from the Finlay Vaccine Institute (IFV) who lead this project, they bet―as they themselves have explained―, after the structure of the virus was known in the scientific world, and doing a meticulous investigation, on the RBD protein (receptor-binding domain) found in the “spike protein,” the “key” to enter the human cell.
*Caption:
SOBERANA 01 VACCINE CANDIDATE
PROJECT LEADERS
THE LEADERS OF THE SOBERANA 01 PROJECT WERE THE FIRST VOLUNTEERS VACCINATED BEFORE STARTING THE CLINICAL TRIAL THAT BEGAN ON AUGUST 24, 2020.
Dr. C. Vicente Vérez Bencomo
General director of the Finlay Vaccine Institute . Cuban scientist with a nationally and internationally recognized trajectory, he is the main author of the synthetic antigen vaccine against haemophilus influenza type b (Hib). He graduated as a chemical engineer from the Moscow Lomonosov Institute, State Doctor by the Orleans University (France, 1983), Honorary Doctor of the Canadian Quebec University in Montreal and Member of the Academy of Sciences of Cuba, among others. He received the Knight Order of the Honor Legion, the best known and important of the French distinctions conferred on men and women for extraordinary merits.
Dr. C. Dagmar García Rivera
Director of Research of the Finlay Vaccine Institute. Holder of a degree in Pharmaceutical Sciences from the Las Villas Central University in 1998. Researcher and university professor. Doctor in Sciences.
Yury Valdés Balbín
Assistant director of the. He forms part of the group of authors of the vaccine against haemophilus influenza type B and is part of the research project to obtain conjugated vaccines against streptococcus pneumonia. In 2016 he won the Annual Prize of the Academy of Sciences of Cuba.
***
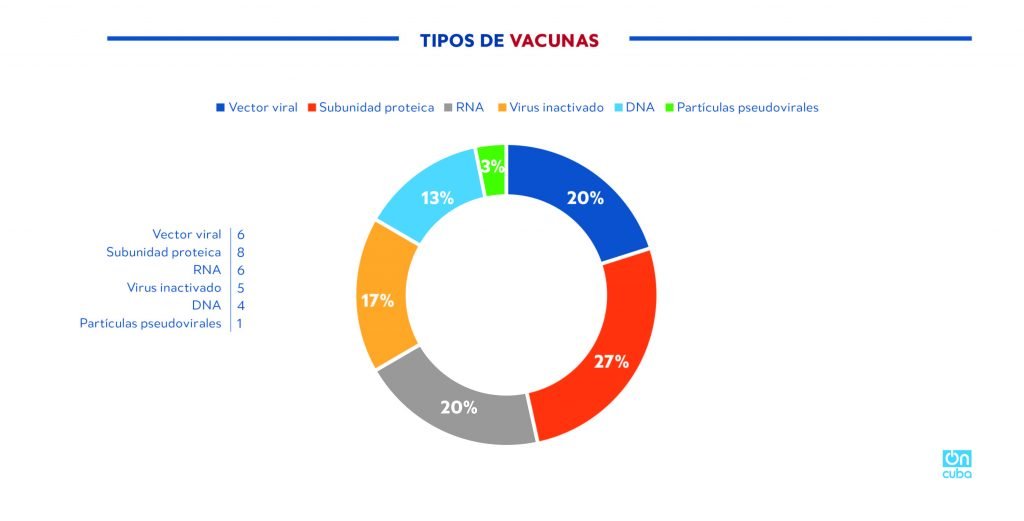
Of the 30 vaccine candidates in the world that are in the clinical trial phase (WHO, August 20, 2020), 8 candidates are of the subunit vaccine type and only two, the Cuban and one developed by China, use the RBD protein, this is most likely due to the fact that its characterization and studies on it are very recent.
*Caption:
Types of vaccines
Viral vector
Subunit protein
RNA
Inactivated virus
DNA
Pseudoviral particles
What are the advantages of working with the RBD?
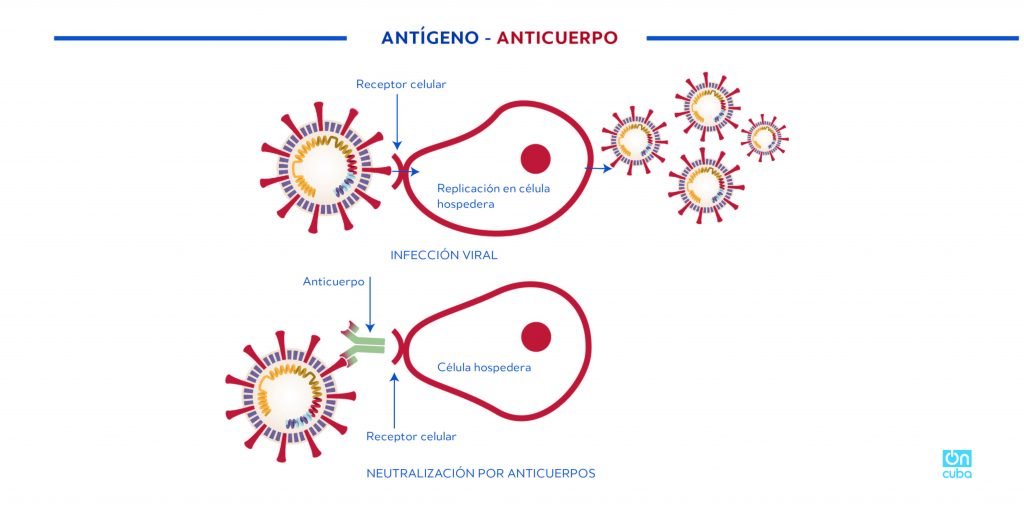
The key and lock theory has been widely explained to understand the entry of the virus into human cells. Putting it in the simplest possible way: the SARS-CoV-2 virus, which causes COVID-19, has a protein in its envelope, the famous spike protein (S), that “binds” to a receptor in human respiratory and digestive cells. Apparently the union between the virus and the cell is determined by a subunit of the S protein known as the receptor binding domain (RBD), that would be the famous “key” that opens the cell for the virus to enter and multiply.
The coronavirus that causes COVID-19 and the one that causes the disease known as SARS, bind to the same cell receptor, however, one of the factors that may be influencing the great viral replication observed in COVID-19 (greater than in SARS) is that this union is more efficient.
Therefore, the RBD protein plays an important role. As this is the active component of the Cuban vaccine candidate, if the preparation is successful, it will generate antibodies against RBD that will block this entry key to the cell and possibly neutralize viral replication.
*Caption:
ANTIGEN-ANTIBODY
Receptor cell
Replication in host cell
VIRAL INFECTION
Antibody
Host cell
Receptor cell
NEUTRALIZATION BY ANTIBODIES
Now then, once this antigen was identified, it was necessary to produce it, purify it and test its ability to generate a protective immune response. RBD is a complex protein, which according to Dr. Belinda Sánchez Ramírez, Director of Immunology and Immunotherapy at the Center for Molecular Immunology (CIM), who led this part of the process, it is necessary to produce this protein with mammalian cell technology, in which CIM has more than 25 years of experience producing recombinant proteins for cancer therapy and the production of humanized monoclonal antibodies.
The CIM not only had to produce the RBD that the vaccine candidate would use, but also seven more recombinant proteins that would be used in analytical techniques necessary as part of the vaccine evaluation process and that Cuba could not buy.
This stage in the development of the vaccine candidate was possible because the CIM has the recombinant DNA technology necessary for the expression of complex proteins in mammalian cells and also has platforms for large-scale mammalian cell fermentation, complex protein purification and for evaluating the identity of recombinant proteins, which allow for the production of RBD that the country would need for a mass vaccination campaign.
In addition to producing the RBD protein, it is necessary to validate the quality of the immune response it induces, both in animals and in humans. This also requires highly specialized analytical techniques in which the CIM has long experience.
Other components of the vaccine
However, this small molecule alone could not be a vaccine. These subunits (in this case RBD) must be “presented” to the immune system and a stable formula must be achieved, which would achieve high immunogenicity and which, in addition, is safe and implies few associated undesirable effects.
That is why the Cuban scientists have explained that they used the conjugation between the RBD with a platform that has been widely studied by the Finlay Vaccine Institute. This platform, which seeks to “present” adequately the RBD to the cells of the immune system, stabilize the formula and enhance the immune response of the cells of the immune system, has been the outer membrane coat system of meningococcus and alumina; the basis of VA-MENGOC, meningococcal BC vaccine.
The outer membrane coat of the meningococcal bacteria has been widely studied by the Finlay for many years as an enhancer of the innate immune response and, in fact, has been studied by the Institute itself as one of the pre-existing Cuban medicines in the fight against COVID-19 since the beginning of the pandemic.
Alumina, on the other hand, is the adjuvant (a word that comes from Latin and that means “that which assists, that helps,” in vaccines it refers to substances or procedures that, incorporated into the antigen or injected simultaneously with it, make the immune response more effective) most recommended in the production of vaccines for humans, offers stability and increases immunogenicity with very few side effects.
The outer membrane-alumina coat platform, part of the Finlay Vaccine Institute’s production line, is tested, standardized and guarantees production, in case the preparation is successful.
*Caption:
SOBERANA 01 VACCINE CANDIDATE
TYPE OF VACCINES
- Of subunit protein
MAIN COMPONENTS
- SARS-CoV-2 virus RBD protein
- External membrane coat of meningococcus b
INVOLVED INSTITUIONS
- Finlay Vaccine Institute
- Center for Molecular Immunology
- University of Havana
- Laboratory of the Civil Defense
- Biocubafarma
- MINSAP
IN CLINICAL TRIALS, ALSO:
- National Center for Technology
- Pedro Kourí Institute of Tropical Medicine
- CIMEQ
- National Center for Clinical Trial Coordination
- Immunoassay Center
KEY DATES:
- May 19: start of design
- July 17: production of first two lots (with different doses)
- August 3: delivery of application approval document for clinical trials in humans
- August 4: release of the lots
- August 10: technical discussion with CECMED
- August 13: CECMED authorization to start clinical trials in humans combines phases I and II
- August 24: first immunization of clinical trial participants
Having achieved, in 7 weeks, the design of a vaccine candidate and the production of lots of vaccines for the start of clinical trials is a great scientific achievement, and in addition to the will, intelligence and dedication of its creators and participants, it has been possible because Cuba has a powerful and undeniable biotechnological development and a pharmaceutical and health system with an experience that has made it possible to use its lines of research and production, the accumulated knowledge, in the solution of a serious global health problem, within the reach of very few biotechnology-pharmaceutical industry and health systems in the world.
*Caption:
AFTER A VACCINE CANDIDATE HAS BEEN DESIGNED IT MUST FOLLOW THE FOLLOWING PHASES
PRECLINICAL TRIALS
- Evaluation of immune response in lab animals
- Evaluation of safety in lab animals
PHASE 1
SAFETY
The vaccine candidate is administered to a small number of people to evaluate safety and ability to produce immune response.
PHASE 2
IMMUNOGENICITY
The vaccine candidate is administered to hundreds of persons divided into groups according to certain characteristics, for example age, to assess the vaccine’s performance in different groups. The immune response and safety is evaluated
PHASE 3
EFFICACY
The vaccine candidate is administered to thousands of persons and the efficacy will be evaluated compared to the placebo group.
*This stage will determine if the vaccine is a protector, the less frequent side effects are evaluated.
APPROVAL
Each country’s regulatory agencies review the results of the trial and decide if they approve or not the vaccine for mass use in humans.
Notes:
* During a pandemic a vaccine can be approved for emergency use before being officially approved.
* Once a vaccine has been authorized it continues being monitored.
Source: The New York Times
Preclinical trials
Dr. C. Dagmar García Rivera, director of research at the Finlay Vaccine Institute and one of the project leaders on whom the design and evaluation of this Cuban vaccine candidate has fallen, explained some of the results of the preclinical trials, an indispensable stage to go on to test the vaccine in humans.
According to the doctor, the preclinical trials in experiment animals (mice and rabbits were used) had two objectives:
- Ability of the candidate to induce an immune response (immunogenicity)
- Evaluation of the safety of the vaccine in animals (using experimental toxicology models)
For this it was necessary to evaluate:
- anti-RBD antibody levels in mice and rabbits
- ability of these antibodies to inhibit the interaction between RBD and the ACE2 receptor.
- the neutralizing capacity of these antibodies against the live virus. *
- the T cell response
It was also necessary to develop hamster infection models to evaluate the protective capacity. *
* To work with the live virus, it is necessary to have laboratories with a high level of biosafety. In Cuba, the only one where this work can be done is the laboratory of the Civil Defense Research Center.
Two doses (0-14 days) were inoculated in the animals. Some results:
- It was observed that there was production of anti-RBD antibodies as of the 7th day after the first immunization (According to the doctor, this is not a usual result, since this response is often more delayed, it could be attributed to the great immunogenic capacity of the platform of the outer membrane coat)
- Between days 21 and 28 after the first immunization, the highest antibody titers are observed in immunized animals.
- The inhibitory capacity of the antibodies in the RBD-cell receptor interaction was studied at 7-21-28 days, finding the best results between 21 and 28 days
- Regarding the response of T cells, a predominance of the Th1 pattern was demonstrated (the most desired to achieve a protective immune response)
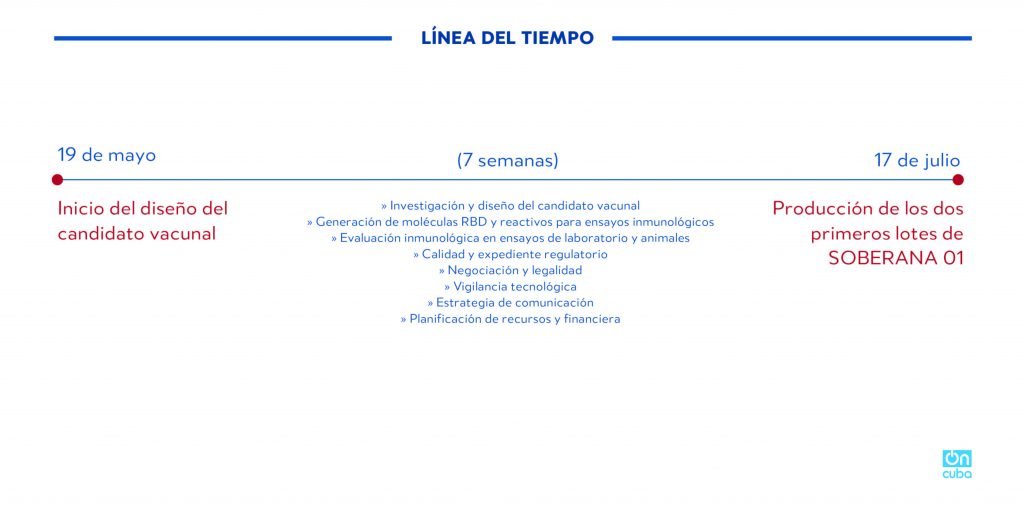
*Caption:
TIMELINE
May 19
Start of vaccine candidate’s design
(7 weeks)
>> Research and design of vaccine candidate
>> Generation of RBD molecules and reagents for immunological trials
>> Immunological evaluation in lab tests and animals
>> Quality and regulatory document
>> Negotiation and legality
>> Technological surveillance
>> Communication strategy
>> Planning of resources and finances
July 17
Production of first two lots of SOBERANA-01
Authorization to start clinical trials in humans:
This is a very important step in the development of vaccine candidates. To get here, the candidate must have fulfilled a series of requirements and the quality must have been checked in each of the steps. This authorization is granted by Regulatory Agencies in each of the countries and they are usually extremely rigorous. In Cuba, it is granted by CECMED: Regulatory Authority for Medicines, Equipment and Medical Devices of the Republic of Cuba.
CECMED is certified by the Pan American Health Organization (PAHO) for the prequalification of vaccines. It was the first authority in Latin America to obtain the status of Competent Authority for the vaccine regulatory system granted by the World Health Organization (WHO). Since 2010, it has maintained the status of PAHO Regulatory Authority of reference, level 4 (the highest level granted), which entails a systematic evaluation to maintain it.
CLINICAL TRIALS
What is starting today in Cuba?
Since August 13, the regulatory entity (CECMED) approved phase 1-2 clinical trials for the Cuban SOBERANA-01 vaccine candidate. As of that moment the preparation of this trial began from the clinical point of view.
As I have already explained, phases 1 and 2 of the clinical trials have the main objective of evaluating the safety and immunogenicity of the vaccine candidate, while phase 3, which is carried out in a considerably greater number of people, has the objective of evaluating the efficacy.
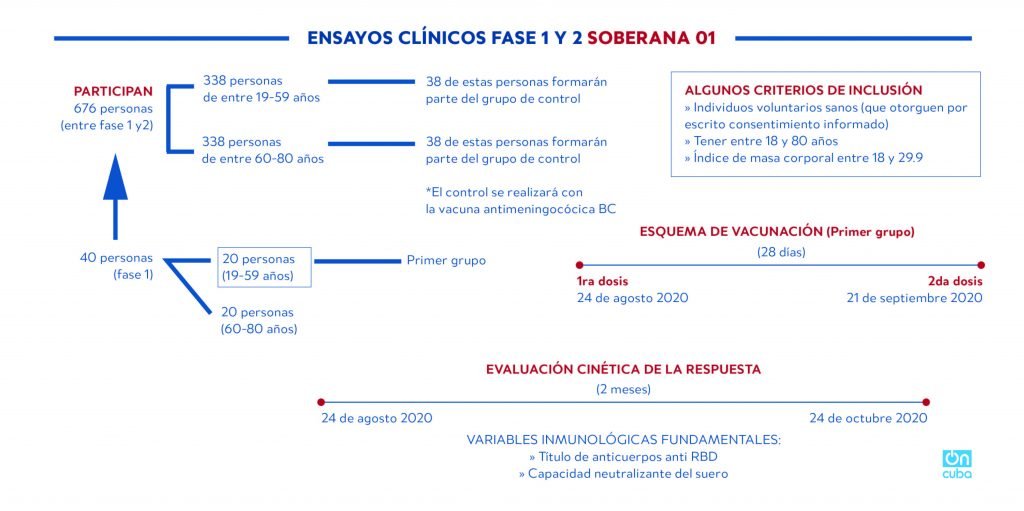
The phase of clinical trials with the vaccine, identified by the acronym FINLAY-FR-1, should begin today, Monday, August 24, and will be administered to a group of 20 people between 19 and 59 years old. A week later, the trial will be applied to another twenty volunteers between 60 and 80 years old.
In the second phase, scheduled to begin on September 11, the sample will be expanded to 676 volunteers, including 40 from the first.
*Caption:
PHASE 1 AND 2 CLINICAL TRIALS SOBERANA-01
PARTICIPANTS
676 persons (between phase 1 and 2)
338 persons aged between 15 and 59
338 persons aged between 60 and 80
38 of these persons will form part of the control group
38 of these persons will form part of the control group
* The control will be carried out with the meningococcal BC vaccine
SOME OPINIONS FOR INCLUSION
>> Healthy volunteers (who grant their consent in writing)
>> Aged between 18 and 80 years
>> Corporal mass rate between 13 and 29.9
40 persons (phase1)
20 persons (19-59 years old)
20 persons (60-80 years old)
First group
Vaccination outline (First group)
(28 days)
1st dose
August 24, 2020
2nd dose
September 21, 2020
KINETIC EVALUATION OF RESPONSE
(2 months)
August 24, 2020
October 24, 2020
Fundamental immunological variables
>> Anti-RBD antibody titer
>> Serum’s neutralizing ability
Some aspects to note:
Normally in this type of trials a placebo group is used; this means that a substance is inoculated that does not induce the specific immune response that is sought in the trial. The placebo can be a saline solution, a vaccine for another disease, or some other substance. In the case of the Cuban clinical trial, the meningococcal BC vaccine will be used as a placebo, which will not stimulate the specific response of anti-RBD antibodies to the SARS-CoV-2 virus, but which contributes to improving innate immunity.
The urgent need to obtain a preventive vaccine against COVID-19 has led scientists and regulatory bodies to modify their work schedules without sacrificing the safety of vaccine candidates. For this reason, phases of clinical trials have been combined as results are obtained. Dr. C. Vicente Vérez Bencomo, general director of the Finlay Vaccine Institute has explained that the same will be done in the Cuban case, which is why the clinical trials whose first immunization is carried out today will include study phases 1 and 2, and as the results are obtained and measured, it will advance to phase 3, which is where it will finally be possible to conclude if SOBERANA-01 is an effective vaccine.
The Cuban strategy against COVID-19 and Biotechnology
Despite the outbreak being experienced in Cuba, after having reached very low numbers of contagions, it can be said that the Cuban strategy against COVID-19 has been successful so far. Until yesterday (August 23), the Cuban health authorities reported a total of 3,682 positive cases, 91 deaths, and 3,044 medical discharges. So far in the world there are approximately 23 million people who have been diagnosed with the virus, more than 800,000 people have died (103.9 deaths per million inhabitants), while so far in Cuba the rate is 8 deaths per million inhabitants. Cuba, according to the death rate for COVID-19, ranks 142 out of the 215 countries that have reported positive cases, that is, 141 countries accumulate more deaths per million inhabitants than Cuba, while 73 have a lower rate. These statistics are influenced by many factors ―I always like to remember that each of these numbers corresponds to a human being―but, without a doubt, the strategies of governments have an important weight. The Cuban has been based on a joint work between the government, the national health system and scientists.
The isolation measures, the active screening, the number of tests carried out, the systematic information, the integration between various areas of science, the authorities’ correct risk perception, the action protocol for medical intervention (which includes several Cuban medicines), have been the keys to being in a “quite favorable” situation so far, if compared to the region (current epicenter of the pandemic) and in the midst of a serious economic crisis that makes daily life very difficult for the vast majority of Cuban men and women.
The Cuban pharmaceutical industry continues to develop specific drugs against COVID-19 that contribute to reducing the mortality rate, while other vaccine candidates are being developed by both the Finlay Vaccine Institute itself and the Center for Genetic Engineering and Biotechnology (CIGB). The evidence is clear and irrefutable; for a small archipelago, blockaded, underdeveloped and with a structural crisis of its economy dating back years, it would be unthinkable to achieve these results―in this record time or at any other time―if it did not have a solid and sustained development in the Cuban biopharmaceutical industry, which has been the political will to prioritize and maintain it.
Why has Cuban biotechnology been successful against COVID-19? (I)
COVID-19 vaccines in the world
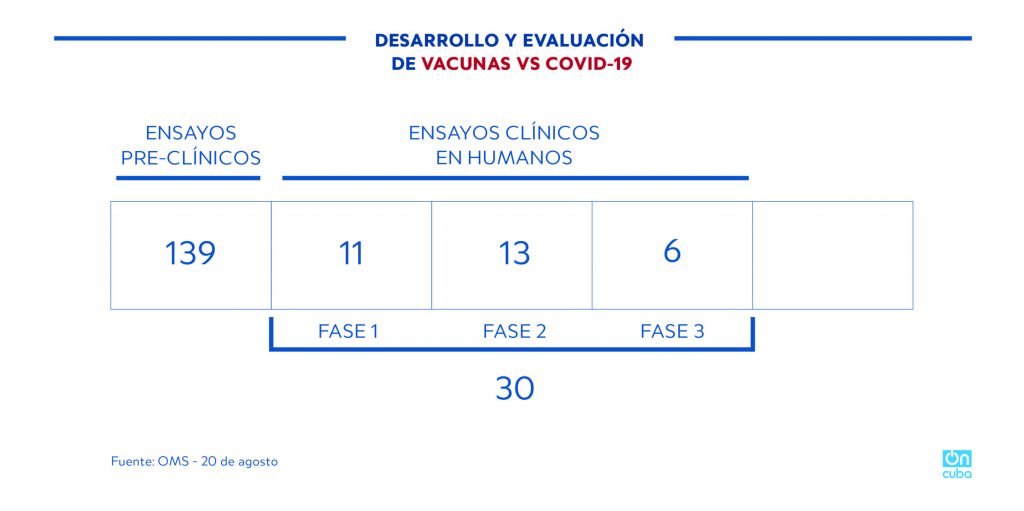
According to the WHO, around 200 COVID-19 vaccine candidates are being developed, of them only 30 (data from August 20, 2020) have authorization to carry out clinical trials in humans. Among these, only six (reported by WHO) are in phase 3 clinical trials (developed by China, the United States, the UK and Germany).
The global race to find a preventive vaccine and specific drugs for the treatment of COVID-19 has been marked by two positive elements: first, by an unprecedented generation of information about a disease and the virus that produces it over a very short period of time. Secondly, and even more importantly, due to the fact that this information has been freely shared among the scientific community in real time, thanks to platforms that were created for that purpose.
*Caption:
DEVELOPMENT AND EVALUATION OF COVID-19 VACCINES
PRECLINICAL TRIALS
CLINICAL TRIALS IN HUMANS
PHASE 1 PHASE 2 PHASE 3
Source: WHO – August 20
*Caption:
COVID-19 VACCINE PROJECTS IN HUMAN CLINICAL TRIALS
DEVELOPER/MANUFACTURER
COUNTRIES
EEUU = United States
Alemania = Germany, China, United States
Alemania = Germany
Korea = South Korea
Japón = Japan
Corea = South Korea
Belgica = Belgium
Rusia = Russia
Italia, etc = Italy, Germany, Belgium
Australia, etc = Australia, South Korea
Taiwan, etc = Taiwan, United States
Francia, etc = France, United States, Austria
Canadá = Canada
Source: WHO – August 20
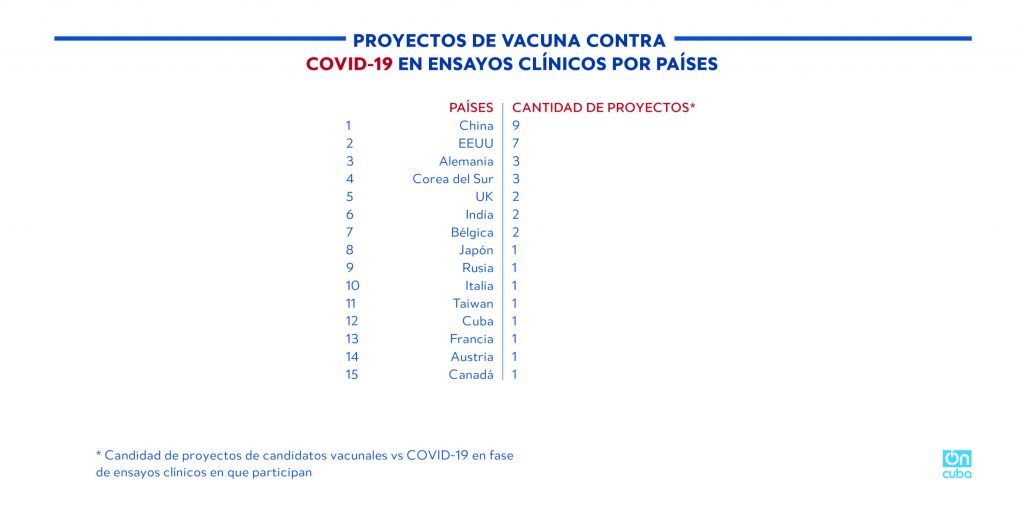
Cuba is among the 15 countries that have proposed vaccine candidates that are in clinical trials, it is the only underdeveloped country and the only one in Latin America on the list.
*Caption:
COVID-19 VACCINE PROJECTS IN CLINICAL TRIALS BY COUNTRY
COUNTRY
AMOUNT OF PROJECTS*
* Amount of COVID-19 vaccine candidate projects in clinical trial phase in which they participate
Why is it important for Cuba to have its own COVID-19 vaccine?
A few days ago, when we suspected that something was happening in Cuba with a vaccine project that had not been mentioned in the press, but some photos and tweets of happy scientists had been “leaked,” a friend—an impeccable journalist—asked me what I thought about this. Smart as she is—word had come out that Cuba could produce the Russian vaccine; the Chinese, with whom the Cuban government and the scientific community have strong links, had also announced their vaccines in phase 3—she asked me: why would it be important for Cuba to have its own vaccine if it can produce others? I must confess that at that time I told her: more than an economic question (which it also is) it’s a question of sovereignty.
The fact that Cuba has its own vaccine and specific drugs against the coronavirus that has caused this pandemic allows for, first of all, the ability to protect its population without depending on any agreement―of course, all possible agreements on health issues are welcome; but it also places Cuba in another place on the map of the pharmaceutical and biotechnology industry, a place logically occupied by highly developed countries. Of course, achieving control of a pandemic of this magnitude directly influences the economy of any country and makes it possible to be in a position to start making progress in recovering from the deepening economic crisis that already existed in the Cuban case.
The ability to respond, to take advantage of accumulated capacities, the mechanism between the different scientific and health centers, the ability to design and set up strategies in record time, the use of advances in different lines of research and production and the ability to adapt them to a new and dangerous circumstance; it grants a multiplied capacity to face possible new epidemics and emerging health problems. It is as if, speaking in immunological language, Cuban science had been vaccinated, and set the line of immune memory necessary to react better and faster to new risks.
I hope that all the vaccines that are being developed are effective, I hope that Soberana-01 is among them, and hopefully some other Cuban candidate as well, I hope that access to the vaccine―whatever its origin―is universal and free as it will be in Cuba.
Until the clinical trials are finished, we will not know if this vaccine will be effective, it’s not about celebrating ahead of time, but with the path that has been traveled to get here, the women and men who do science in Cuba have again already made history.
***
Sources:
* Cuban scientists’ participation in the Mesa Redonda program on Cuban television broadcast on August 20, in which the following participated: Dr. C. Vicente Vérez Bencomo, general director of the IFV; Dr. C. Belinda Sánchez Ramírez, director of immunology and immunotherapy at CIM; Dr. C. Dagmar García Rivera, IFV research director; Ing. Yaquelín Rodríguez Valdés, deputy director of CECMEC; Yury Valdés Balbín, deputy director of the IFV.
* WHO official site
* PAHO official site
* Section dedicated by The New York Times to COVID-19