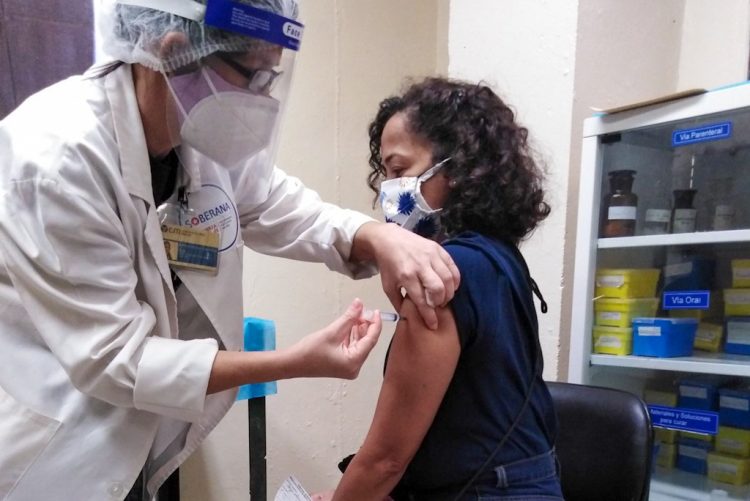
Scientists from the Finlay Vaccine Institute and the Center for Molecular Immunology are forming part of the 150,000 volunteers in an “intervention study” with the Cuban Soberana 02 COVID-19 vaccine candidate, which began this Monday in Havana.
The new study, approved this weekend by the Center for State Control of Medicines, Equipment and Medical Devices (CECMED), aims to evaluate the effects of vaccination in population sectors with high “risk of infection, disease and spread of the pandemic,” according to Prensa Latina (PL) news agency.
Este grupo está formado por los primeros trabajadores que se vacunarán con #Soberana02 en el marco del ensayo de intervención controlada. Ellos son investigadores, ingenieros, chóferes, abogados y personal de servicios que han apoyado el desarrollo de nuestras vacunas.#CubaViva pic.twitter.com/yd7ovFl5rN
— Instituto Finlay de Vacunas (@FinlayInstituto) March 22, 2021
Developed by the state-run Finlay Institute, Soberana 02 became the first vaccine candidate in Latin America to begin phase III of clinical trials on March 8, with 44,010 volunteers from eight municipalities in Havana.
After 15 days since the start of that trial and still without preliminary results, the new study has begun, having among its volunteers the health personnel, as well as from the pharmaceutical and biotechnological sector of the Cuban capital and other sectors defined by the Ministry of Public Health, among the which are those in charge of the development of vaccine candidates and other drugs to prevent and face COVID-19 on the island.
Inicia en el CIM proceso de vacunación de Soberana02.
Una de las primeras vacunadas es la Dra. Tays Hernández, investigadora del Centro, al frente del equipo de seguimiento de la respuesta inmune de los sujetos vacunados de las Soberanas. #VacunaCubanaCovid19 https://t.co/EV0ce87vcB pic.twitter.com/LHfkxMEVci— Centro de Inmunología Molecular (CIM) (@cim_cuba) March 22, 2021
The intervention will serve to demonstrate the direct and indirect consequences on the prevention of symptomatic COVID-19 and to evaluate the effect on the prevention of severe disease, as well as to assess the results of Soberana 02 on the mortality caused by the contagious disease, PL indicated.
Like the phase III trial already underway, this new study aims to document the safety profile of the vaccination scheme with two doses of this candidate and a third booster with Soberana Plus, another Cuban vaccine candidate aimed at increasing immunity against the SARS-CoV-2 in people already immunized or convalescing from the disease.
At the same time, the third phase of tests with the Abdala vaccine candidate, developed by the Center for Genetic Engineering and Biotechnology (CIGB), also began this Monday in the provinces of Santiago de Cuba, Guantánamo and Granma.
Vacuna cubana Abdala comienza su tercera fase de ensayos el próximo lunes
The necessary doses of Abdala for the study that will involve 48,000 volunteers between 19 and 80 years of age, arrived this weekend in the capitals of those eastern provinces of the island, the Agencia Cubana de Noticias news agency reported.
Cuba is going through its worst wave of coronavirus infections since the beginning of the epidemic in March of last year. With 10 of its 15 provinces and the special municipality of Isla de la Juventud in the epidemic phase and a daily average of between 700 and 900 infections, the island hopes to have a vaccine ready in the coming months to immunize its entire population this year.