Cuban science, particularly that linked to the pharmaceutical industry, has been decisive in the fight against the COVID-19 pandemic on the island and beyond its borders. Its contributions, according to executives and specialists, have been concentrated in three main directions.
In a first work, we addressed the development of medications that seek to prevent vulnerable people from contracting the disease and becoming seriously ill. Drugs such as Biomodulin T and the vaccines to increase people’s innate immunity can be mentioned in this direction.
A second course is that of the products used for the usual treatment of those who become infected with the virus. This is a key line of work since, according to Dr. Eduardo Martínez, president of the BioCubaFarma business group, “patients whose immune system fails to respond effectively to infection can have a viral load up to 60 times greater than that of those who go through the disease asymptomatically or mildly, so it is very important to have antiviral agents that reduce this load.”
Treatment of COVID-19 patients
Recombinant Alpha 2B human interferon
Of this drug, a pioneer in the Cuban biotechnology industry, “there is plenty of evidence of its antiviral action” and it has once again proven its worth against the new coronavirus, even before the report of its first cases on the island, confirmed Dr. Eulogio Pimentel, general director of the Center for Genetic Engineering and Biotechnology (CIGB).
This drug, produced by the CIGB since 30 years ago, started being used from the beginning of the epidemic in China―a country where it is also produced by a joint venture between Cuban and Chinese entities―and since then it has been showing encouraging effects, both reported in international publications as well as proven by its use in Cuba.
According to Pimentel, “in Cuba there are no objections to its use in the sick population” and “its results, combined within the protocol of therapeutic action established by the Cuban Ministry of Public Health, are surprising due to the magnitude of the efficacy obtained.”
“More than 92% of virus-positive patients in the country have used interferon in their therapy, and the data from the results obtained show that the number of patients becoming seriously ill is practically reduced twofold, compared to those who haven’t received this therapy in Cuba,” explained to the press the director general of the CIGB, a center of proven reputation that since the beginning of the pandemic has launched a dozen projects associated with different stages in which action can be taken from the therapeutic point of view against COVID-19.
“Between 15 and 20% of patients in the world evolve towards a critical and serious stage of the disease, while in Cuba it has been less than 5% in the case of patients treated with Heberon (trade name of the Cuban interferon),” which, in the opinion of the specialist, evidences its “indisputable capacity to drastically reduce the number of patients that evolve towards becoming seriously ill.”
To this is added that while in the world the fatality rate is around 7%, on the island the patients treated with Heberon are below 1%, which, according to Pimentel, “is not the only consequence of the use of interferon,” but of the applied therapeutic protocol “in which interferon is playing a key role.”
However, despite these evidences, the official commented that “its efficacy” and data supporting it continues being studied, while a clinical trial is being developed that combines two interferons, the aforementioned Alpha 2B and gamma, about which “I cannot advance anything yet, but with which we feel very motivated.”
Inventories and demand for Cuban interferon
Due to its efficacy, Cuban interferon has generated great international interest and BioCubaFarma has already been contacted by more than 80 countries, “to some of which it is exported, in other cases the contract process is underway, and with others there is an exchange of information for a possible contract,” stated its president, Dr. Eduardo Martínez.
However, the executive assured that the island’s pharmaceutical industry’s policy is “not to export any medicine if there is a risk of it being lacking in the country, something that we take very much into account in the case of the new coronavirus pandemic.” And he emphasized that “the priority is to guarantee the drugs for the worst scenario that we could have, for the number of patients that we have calculated that there could be in that case, and that amount of drugs is set aside.”
In addition, he affirmed that in the case of interferon, the current international demand does not endanger existing inventories in Cuba or its use in Cuban COVID-19 patients, a fact that was confirmed by the director general of the CIGB, who assured OnCuba that the island today has “100% coverage of recombinant Alpha 2B human interferon for the treatment of SARS-CoV-2 in the country.”
Two months ago, Pimentel had replied to our media that his center then had an inventory of finished product “for the cases we estimate may appear in Cuba in a horizon of between three and six months,” although he did not offer a specific figure in this regard. Now he specified that “assuming that up to this moment we have had half of the patients predicted by the models, in those patients we have used a third of the amount that we have available.”
“We have enough inventory of interferon Alpha 2B to treat all patients who could still be infected according to what the prognostic models foresee and, even so, we have much more inventory in case it is necessary to use it in Cuba and to share it with other countries,” he affirmed.
Other antiviral medicines
Chloroquine
Beyond interferon, the island also produces other drugs that are used in the treatment of COVID-19 patients. This is the case of chloroquine, which until now has been used against other diseases such as malaria, rheumatoid arthritis and autoimmune lupus disease, and which since the pandemic spread has become part of the protocols established in several countries, including Cuba, for its immunomodulatory nature and also its possible antiviral effect.
Kaletra (lopinavir and ritonavir)
Furthermore, even though it is not yet manufactured, the pharmaceutical industry is working on the development of its own version of Kaletra, a combination of two antiretrovirals―lopinavir and ritonavir―originally created to treat AIDS patients, which until today is imported by the island and also plays an important role in the Cuban protocol against COVID-19.
The first trials of the Cuban prototype of this drug have yielded “satisfactory results,” according to recent statements by Adalberto Izquierdo, one of the leaders of the investigation carried out by the company MedSol, who, however, explained that for its large-scale production, “adaptations in Cuban technology” are required in the absence of an important equipment for the manufacture of Kaletra on the island for the time being.
Cuba developing its own version of an antiretroviral for use in coronavirus patients
New drugs
Along this line, the president of BioCubaFarma also pointed out that his business group is working “on two drugs based on peptide molecules, which have already shown an antiviral effect in in vitro experiments with other coronaviruses, but we have found important evidence that they can inhibit the replication of this virus.” In this regard, he explained that Cuba has established “collaboration with China, in laboratories there, to already test them on the new coronavirus to verify the effectiveness of these molecules.”
Treatment of critically and seriously ill patients
Among the treatments for COVID-19 patients, Cuban science has paid special attention to those who, for different reasons, enter a serious and critical stage.
“At an international level, a high percentage of critically ill patients die, and in Cuba we have achieved better indicators with the protocols and medications that we have tested,” said Dr. Eduardo Martínez. The official specified that for this BioCubaFarma proposed to the island’s health authorities “the use of our medicines that were used in the treatment of other diseases, but that their mechanism of action and the behavior of this virus, in particular the so-called ‘cytokine storm’ that leads patients to become seriously ill and even to the death of patients, we believe they could be effective.”
After the first assessments, which left “favorable evidence in a group of patients,” the Cuban Ministry of Public Health (MINSAP) “decided that they should be extended to the entire country, and that is the process we are in,” Martínez said.
CIGB 258
One of these products is known as CIGB 258, developed by the Center for Genetic Engineering and Biotechnology, from which it takes its initials. The project, led by Dr. María del Carmen Domínguez, was initially aimed at treating chronic autoimmune inflammatory diseases, but it has found a very promising niche against COVID-19, since it contributes to controlling the magnitude of the inflammation produced by the disease and does not generate immunosuppression.
“It is a project we are very focused on and that keeps us optimistic, and although its results are not yet conclusive, they do draw a lot of attention,” said Dr. Eulogio Pimentel.
“For example,” explained the director general of the CIGB, “in the United Kingdom the rate of patients who die after becoming critically ill is around 67%, in the United States, over 80%, while in Cuba, of the 22 patients evaluated in a study done until the end of April, only 11% had died. Of these patients, only one remained serious, 11 went from seriously ill to a care state and six were discharged from hospital. These are not conclusive data, as this is an ongoing investigation, but they are very stimulating.”
From the Center for Molecular Immunology
Parallel with the CIGB, the Center for Molecular Immunology (CIM) has also focused part of its efforts on drugs that help patients with an advanced stage of COVID-19. In this regard, its director of clinical research, Dr. Tania Crombet, commented that one of the studies “that we started most quickly was a research protocol associated with a monoclonal antibody that has a very important role in the contraction of the inflammatory response, in the reduction of proinflammatory cytokines that have a relevant role in the disease.”
Itolizumab
Itolizumab is a humanized monoclonal antibody with more than 20 years of clinical research and produced in Cuba since 2014, which is used to treat other conditions such as rheumatoid arthritis. In the case of COVID-19, it acts in the phase “where the damage is caused by the exaggerated response of the immune system to the virus’ enormous capacity to divide,” and its use could help to “stop in time” the consequences of the cytokine storm, a life-threatening immune reaction.
Its study has covered more than 65 patients from some 10 hospitals throughout the island, classified as critical, serious and in a care state with a high risk of aggravation for being vulnerable patients due to their advanced age and/or for presenting other conditions such as high blood pressure, diabetes , asthma, chronic obstructive pulmonary disease, or having suffered heart attacks or cerebrovascular accidents, according to Dr. Crombet, who said that in a first cut of the research, a percentage was reached of 40% of patients who left intensive therapy or who were even discharged.
“These figures improve according to the patient’s condition at the time of starting treatment; in the case of care patients, the rate is 80%,” said the specialist, who explained that “data is still being collected and an analysis of many parameters is being made to reach definitive conclusions on the effectiveness of the monoclonal antibody in reducing proinflammatory cytokines and the improvement of patients.”
Cuba uses monoclonal antibody to control “cytokine storm” in COVID-19 patients
Recombinant human erythropoietin
In addition, the CIM also contributed to the treatment in the severe phases of COVID-19 the recombinant human erythropoietin, “a medicine that has been registered for many years in Cuba and is used in anemic patients with kidney failure, in premature children’s anemia, and in patients with AIDS or cancer with anemia,” said Crombet.
Regarding the reasons for its use during the pandemic, the director of clinical research at the CIM affirmed that the role of this drug in cytoprotection is known, so the Cuban protocol for care of critically or seriously ill patients included its use “knowing that protection from the ischemic damage seen in this type of patient has to be sought.” Therefore, “the doses that are being used are cytoprotective and will allow improving the degree of hypoxia (absence of sufficient oxygen in the tissues) that this type of patient has when reaching a critical or serious condition,” she said.
For recovery
Although it is the last stage, the recovery of those who have suffered from COVID-19 has not been neglected by the Cuban pharmaceutical industry. Its scientists have worked, and continue to do so, in the development and production of medicines and natural products to recover these people and “stabilize their health in a faster and more effective way,” said Dr. Eduardo Martínez.
“We are rapidly studying the health status of patients once they recover from the disease, and we have seen the possibility of intervening with a group of medications,” said Martínez, who explained that work is underway in the manufacture of “a molecule to increase the presence of white blood cells, of the defense cells of the organism in these patients, because the immune system is really weakened.”
For her part, Dr. Tania Crombet pointed out that the CIM has a research protocol “associated with regenerative therapy, for use in patients who leave hospital centers as cured patients, in which a medicine whose commercial name is Leucosin is used.” It is, she said, “a factor that stimulates granulositic colonies, a drug that, due to its mechanism of action through stem cells, could contribute to regenerating fibrous respiratory tissue that may remain as a sequel and thus improve the respiratory function that is often left with restrictive disorders after suffering from the disease.”
To the aforementioned, the president of BioCubaFarma added that his business group is also working on the production of nutritional supplements, capsules rich in omega 3 and omega 6, propolis tablets, “products that as a whole can help restore immune capacity and strengthen the health of people who have suffered from COVID-19.”
Vaccine
Finally, Martínez confirmed that Cuba is working “in an accelerated way” to achieve a specific preventive vaccine for this virus, along with dozens of other projects that are being carried out around the world in this direction. “We have several vaccine candidates,” he said, “and we soon plan to report the results we are obtaining with them.”
“So far we are very satisfied with the results achieved with the drugs developed and with the research underway to deal with this disease, and we continue working with the MINSAP to improve the treatments and the health of the patients. That is our priority right now,” he concluded.
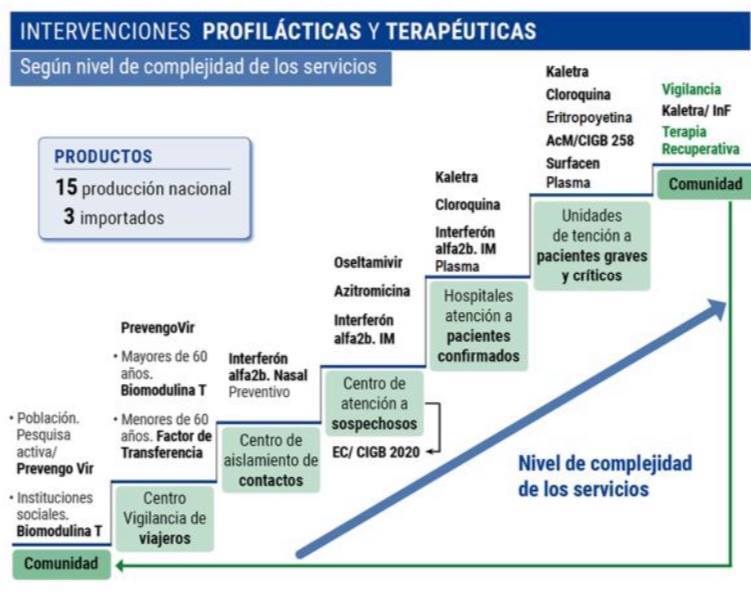
*Caption:
PROPHYLACTIC AND THERAPEUTIC INTERVENTIONS
According to level of complexity of services
PRODUCTS
15 national production
3 imported
- Population
Active screening/
Prevengho Vir
- Social institutions.
Biomodulin T
Prevengo Vir
- Over 60.
Biomodulin T
- Under 60.
Transference Factor
Interferon Alfa 2b. Nasal
Preventive
Oseltamivir
Azithromycin
Interferon Alfa 2b. IM
Kaletra
Chloroquine
Erythropoietin
AcM/CIGB 258
Surfacen
Plasma
Surveillance
Kaletra/ InF
Recuperative Therapy
Community
Center for Surveillance of travelers
Center for isolation of contacts
Center for care of suspects
EC/ CIGB 2020
Hospitals for care of confirmed patients
Care units for serious and critical patients
Community
Level of complexity of services










