Just a few days ago, the announcements about the efficacy of Cuba’s two main COVID-19 vaccine candidates, the result of the third phase of their clinical trials, made headlines. First, 62% of Soberana 02 was reported in its two-dose scheme — one of the two administered in the study —, and then that of the three doses of Abdala, at a remarkable 92.28%.
Since then there have been many reactions and comments inside and outside the island. Satisfaction, pride, joy and even euphoria have prevailed not only among the experts and authorities of the country, but in the general population and also among people from all parts of the world, who see in these drugs an indisputable success of Cuban science and a hope in the face of a disease that has kept the planet in suspense for more than a year and has already taken millions of lives.
However, the incredulity, suspicions, denial and discredit of some have not been lacking either, more for political than scientific reasons, and also those who, from a more measured, more critical stance — in the broadest sense of the word —, and even without ignoring the result, have called for not shouting from the rooftops until there is more data to help unravel and support the announced figures — as has happened with other already registered vaccines —, and for their validation by the international scientific community and global entities such as the World Health Organization (WHO).
After the emotion because of the news, Cuban specialists provided this Thursday more details on the calculation of the efficacy of both vaccine candidates and addressed the steps to follow after revealing that necessary indicator, both for the massive use of the preparations on the island — at a time when the country is reporting the highest numbers of daily infections and active cases of the entire pandemic — as well as for its recognition and commercialization beyond its borders.

Blessed efficacy
The percentage of efficacy is the data that everyone expects from the COVID-19 vaccine, which has taken all the spotlight in the race against the clock to produce immunogens against SARS-CoV-2 and prevent the development of the symptomatic disease as best as possible. Its calculation, which is derived from conducting phase 3 clinical trials with a significant number of people, is not exactly simple and is like the jewel in the crown of the research carried out, after determining the safety and ability to generate immune response in the previous stages of the study.
Thus, the high efficacy reported by the Pfizer-BioNTech, Moderna and Gamaleya (Sputnik V) vaccines, all above 90%, quickly became headlines and mandatory references in the current context. Meanwhile, other internationally recognized and used formulas, such as those of AztraZeneca with the University of Oxford, the single-dose of Johnson & Johnson, the Chinese Sinopharm and the Indian Covaxin stand at more than 70%. Such numbers do not reflect the ability of these preparations to prevent infection, but rather to avoid symptoms of COVID-19 and in many cases decrease — even when they continue to provide protection — against new, more contagious strains of the coronavirus.
As Dr. Eulogio Pimentel, vice president of the state group BioCubaFarma, explained this Thursday on the Mesa Redonda television program, the “vaccine efficacy” is defined by the WHO as the percentage of reduction in the incidence of the disease in the vaccinated subjects with respect to the group given placebo in the studies. This is determined “under the standards and supervision of good clinical practices required by clinical trials” and is expressed mathematically as “one minus the risk of getting sick when you are vaccinated among the risk of getting sick when you are not vaccinated (VE = 1 — Relative Risk = 1 — Risk in the vaccinated person ÷ Risk in the unvaccinated person).”
According to these calculations, that a vaccine is, for example, 66% effective “does not mean that, of every 100 people, 44 will get sick and 66 will not get sick, but that, if they are vaccinated, there is a 66% probability of not getting sick,” clarified the specialist, who pointed out that in the case of “vaccine efficacy,” although it is related to efficacy, it is not measured in a controlled environment like this, but in its open and massive application in society , “which means there is no supervision by the product promoter or the trial’s principal researchers.”
“The less people are able to be immunized in a population, the less the efficacy” of a vaccine is seen, said Pimentel, and stressed the importance not only of getting vaccinated but also of completing the immunization scheme for each vaccine, so as not to leave “a gap for the entry and spread of the virus.”
Coronavirus: eficacia de fórmulas cubanas abre esperanza a primera vacuna latinoamericana
Soberana 02, first cut
Developed by the Finlay Vaccine Institute (IFV), Soberana 02 beat its older sister, Soberana 01, to advance first to phase 2 and then to phase 3 of clinical trials. The latter was held in Havana as of March, with 28-day intervals between each dose, and involved more than 44,000 volunteers distributed in three groups: one who was administered two doses of Soberana 02, the other to whom a third dose was also injected, this one from another candidate in the family: Soberana Plus — also used in studies with convalescent patients — and a third group that received a placebo.
Neither the participants nor the health workers and researchers in the field knew the nominal composition of the groups and it was not until the end of the study that, in order to determine the efficacy, the codes were opened and which volunteers belonged to each one was revealed. This strict protocol, established according to international scientific standards, was also used in the clinical trials of Abdala, the most advanced COVID-19 vaccine candidate at the Center for Genetic Engineering and Biotechnology (CIGB).
In addition, in both cases and following globally established regulations, the efficacy calculation was carried out by a committee of experts who are not part of the vaccine promoter institutions or the teams in charge of research and development of vaccine candidates, but by other scientific institutions such as the Pedro Kourí Institute of Tropical Medicine (IPK) and the Center for Molecular Immunology (CIM), and the Ministry of Public Health (MINSAP).
“From the moment we considered that we had reached the number of cases that would allow an intermediate analysis of the efficacy evaluation to be carried out, it was decided to close the database and hand it over to this independent committee, which first made an analysis by groups of the statistical difference, without knowing what each of the three groups was. They were blinded in this regard,” explained Dr. Vicente Verez, director of the IFV, at a press conference about the particular case of the cut of two doses of Soberana 02.

“On Thursday of last week this committee met with us and informed us that they found statistically significant differences between the groups and that was the green light to hand over the code for each group, which was sealed, of course, since the trial began, and only then it was possible to know who is part of each group,” commented the leader of the development team of the Soberana family of vaccines.
Verez added that “based on that analysis it was possible to verify how each group behaved” —in the case of the one that includes the application of a third dose of Soberana Plus for this intermediate cut of efficacy, the results were only evaluated up to the second dose of Soberana 02— and that “while the risk of contracting the symptomatic disease among those who received a placebo continued to grow over time, the other groups tended to stabilize and decrease the risk.”
Certainties and forecasts
The expert carried out a comparative analysis between the immune response registered in the 810 participants in phases 1, 2 A and 2 B of the Soberana 02 clinical trials, and the efficacy calculated now in phase 3, which shows a certain correlation between both indicators — although not exact —, which allows for making forecasts in this regard, something that, he said, has already been noted by other researchers and publications around the world.
“The study revealed that, with a first dose, in the group of 810 people who participated in the first phases, 19.5% exceeded the antibody titer, that is, they developed neutralizing antibodies to the virus,” he explained. “This allowed us to do initial analyzes that pointed to an efficacy in a dose of between 20% and 30%, to prevent the symptomatic disease of COVID-19. Then, the expert committee concluded that the efficacy in one dose is 30%, which means that there is a 30% lower risk of contracting the disease from receiving one dose of Soberana 02.”
“Regarding the results with two doses in the same group of volunteers, it was found that 76.2% exceeded the threshold that correlates to have neutralizing antibody titers. That made us estimate a possible efficacy of between 60 and 70% of the application of two doses in the phase 3 trial. That was what we were expecting. And when the independent committee closed its analysis, it found that the efficacy was 62%, a remarkable result considering that it exceeds the 50% considered by the WHO for a vaccine candidate to be effective, and because it was achieved in a scenario where there is a high presence of the so-called ‘worrisome strains,’ which constituted a great challenge for our study,” he added.
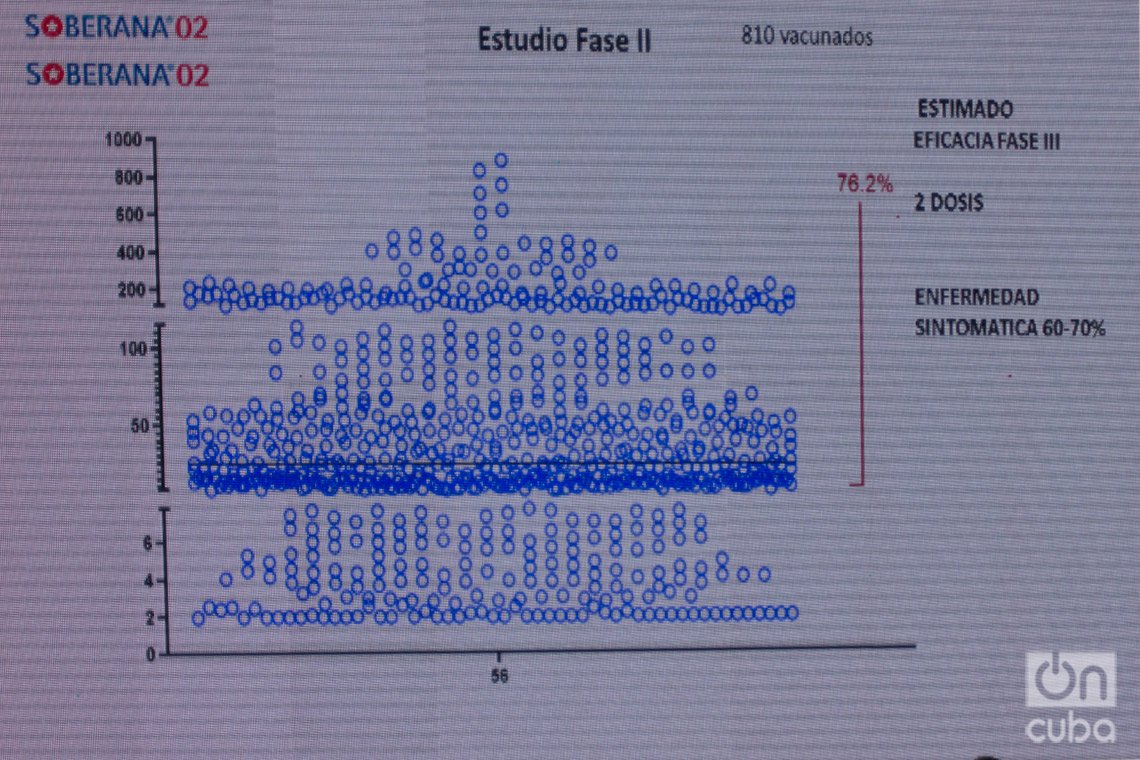
Verez also highlighted that the second requirement established by the WHO to validate a vaccine is that “the sample has sufficient statistical value so that a minimum confidence interval is greater than 30%, and that is also achieved with Soberana 02.” He added that “this was determined based on 111 cases of symptomatic COVID found 14 days after the second dose was administered: 62 cases in the placebo, 23 and 26 in the other two groups. And with these data, when the corresponding calculations were made, they determined the efficacy of the 62% that was already announced. For this reason, Soberana 02, in its two-dose schemle, meets the requirements to be considered an effective COVID-19 vaccine,” which, he said, “is a source of great joy for all of us.”
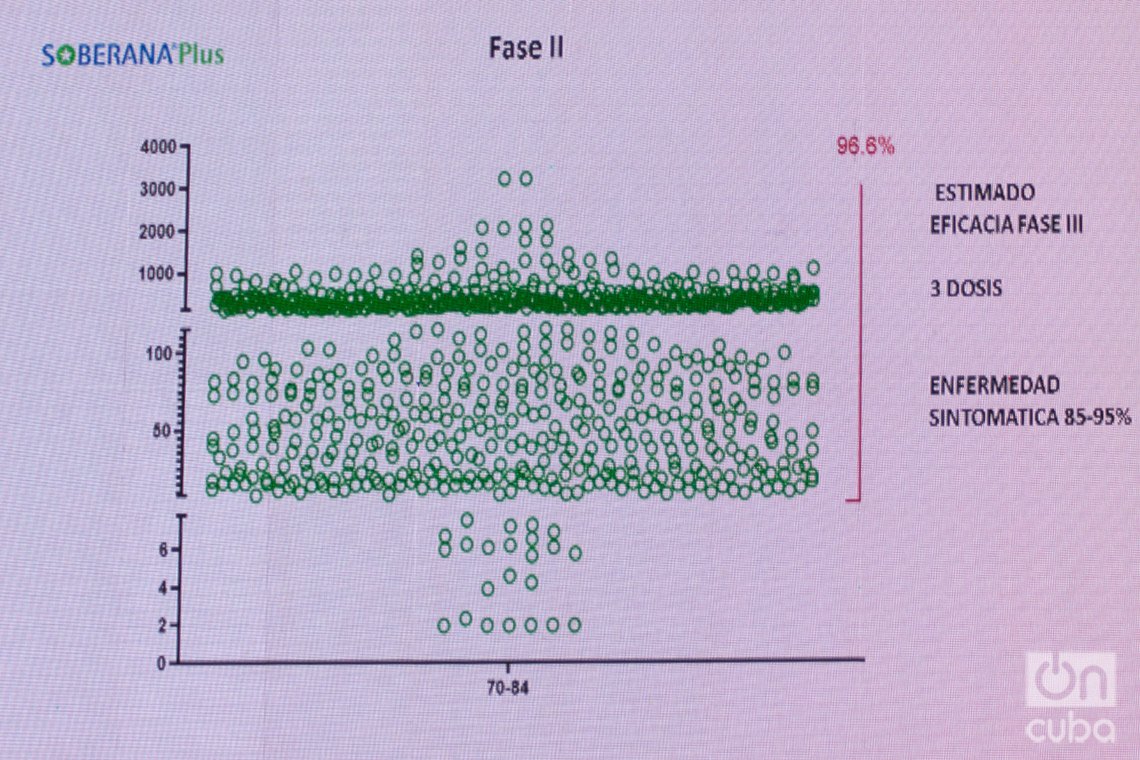
The director of the IFV announced that they are now working on calculating the efficacy of the scheme that includes the third dose of Soberana Plus and said that the researchers under his command expect “high efficacy” from this combination, because 96% of the group studied “correlates with high immunity” and “exceeds the threshold of neutralizing antibodies.” This, he said, is a “very promising” indicator that allows them to make “a forecast with a significant level of approximation,” although, he stressed, “it does not offer an exact or conclusive correlation.” Based on these data, he predicted a possible efficacy of between 85 and 95% for the three-dose scheme.
Abdala, the path
The immunogen named after the character of the well-known epic poem by Cuban poet and national hero José Martí, astonished many when earlier this week its high percentage of efficacy was reported: 92.28. After the intermediate cut of the two doses of Soberana 02, the jump of 30 percentage points with respect to it put the candidate produced by the Center for Genetic Engineering and Biotechnology (CIGB) in the spotlight, to the point of being compared with successful COVID-19 vaccines such as those from Pfizer and Moderna, which are currently used in highly developed countries.
The figure, however, did not come out of the blues and it did not surprise those who developed and always bet on the quality of this candidate, which, biotechnologically speaking, is a subunit vaccine based on the RBD receptor binding site of the S protein of the virus. Meanwhile, the most advanced of the IFV vaccine candidate uses this same technology, but combined with tetanus toxoid, in order to enhance the immune response in the face of a possible infection, which is why it turns out to be a conjugate vaccine.
Unlike Soberana 02, which developed its clinical trials in the Cuban capital, Abdala conducted them in the eastern part of the country. The first two phases took place in the capital city of Santiago de Cuba, while already in the third — and a determining factor to calculate the efficacy — the studies were extended to the provincial capitals of Granma and Guantánamo. There were a total of 48,290 participants in the double-blind, randomized and placebo-controlled trials, in which it was also not immediately disclosed who belonged to which group and a scheme of three doses every 14 days was used.
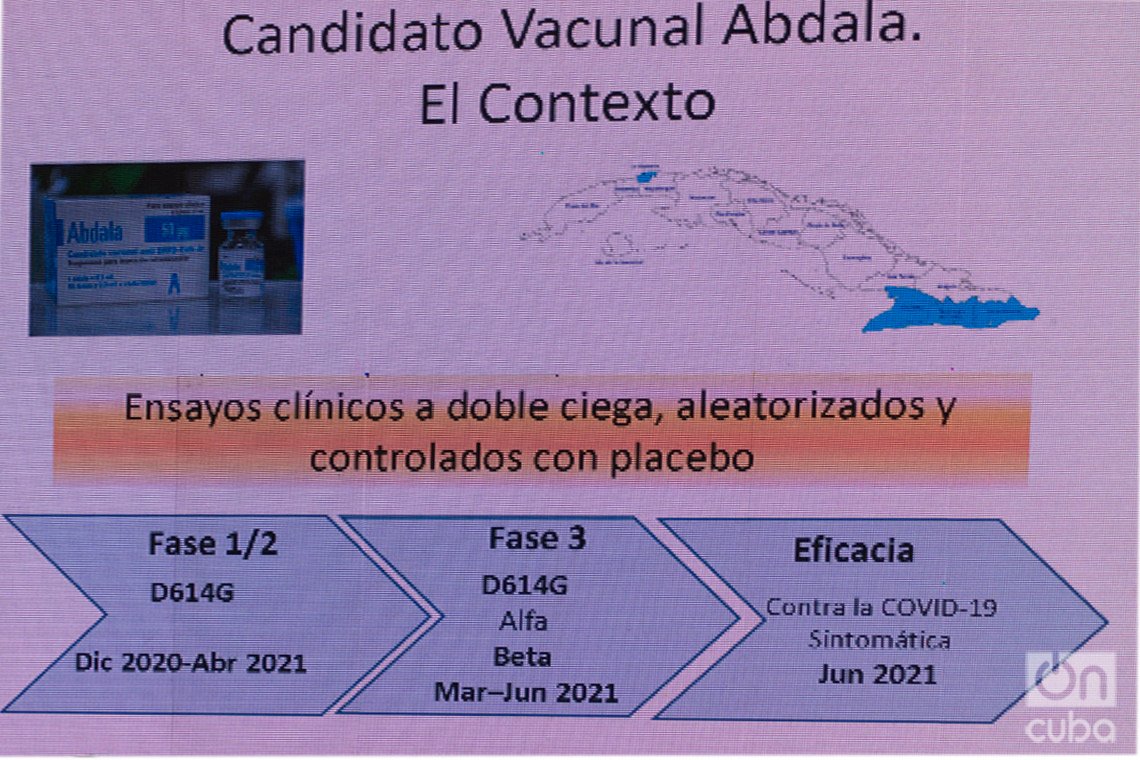
Although the first stages of the study took place in a context in which the predominant variant of the virus in Cuba and in the world was called D614G, as the third phase progressed, the epidemiological scenario changed radically with the spread of new, more contagious strains, which multiplied the infections and incidence rates throughout the island.
“Already when we were conducting the phase 3 clinical trial, and especially at the time when we were collecting the information to determine efficacy, we knew that in the eastern region, as was happening in Havana, other variants of SARS-CoV-2 had already begun to circulate, such as Alpha, identified in the United Kingdom, and Beta, which was identified in South Africa, and which are much more contagious,” Dr. Marta Ayala, general director of the CIGB, explained to the press. She pointed out that, taking this context into account, the Abdala developers think that the efficacy data obtained “in a high probability is also expressing protection against these variants that were mostly circulating.”
Dr. Ayala commented that, according to the studies carried out, the immune response induced by this vaccine candidate “was very good and correlates very positively with viral neutralization,” which is linked to the safety demonstrated from the initial and proven tests in ongoing interventions. “We were able to confirm through an ELISA test from the Roche company that what we had found in terms of the intensity of the response of the vaccinated individuals was really very good and made us foresee that we could also have good results in the evaluation of efficacy. Evaluated by this system, all individuals were positive for the vaccination,” said the specialist.
Data calculated and to be calculated
The increase in incidence and transmissibility in the eastern region, where phase 3 clinical trials were being carried out, made it possible to accumulate symptomatic COVID-19 cases among study participants necessary for the final calculation of the efficacy of Abdala, which was carried out by independent groups of experts and statisticians “as was established in the protocol,” confirmed the director of the CIGB.
“We had planned I and II interim cuts, but based on the analysis of the epidemiological situation and the high incidence that occurred in the eastern provinces, we were able to accumulate the number of events that we expected for the final analysis: 153. Of them, 142 were in placebos and 11 in vaccinated volunteers, which gave us 92.28% efficacy. In addition, the limits of the confidence interval are between 85% and 95%, which is very significant and gives us the certainty that we are really in the face of a very reliable figure,” said Marta Ayala, who said that based on the data obtained “the risk of getting sick for vaccinated individuals is really very low,” while “in placebos, as time progresses, events accumulate and risk increases.”
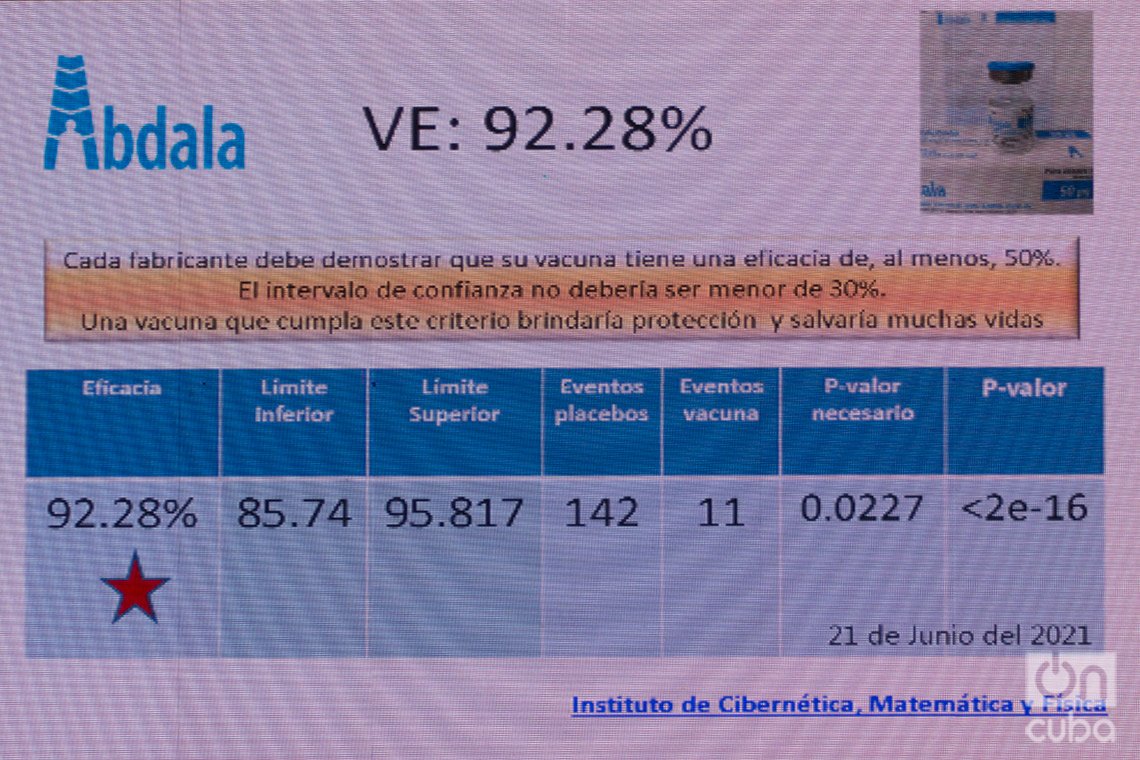
“With this 92.28% efficacy, we are among the best vaccines in the world,” said the scientist, who highlighted satisfaction with the result achieved after months of intense work and reiterated that the announced figures meet the requirements of the WHO and other international organizations and to consider that a vaccine candidate is really effective.
Such success, however, does not mean the end of the road, as research continues with the aim of studying other variables and revealing new data on the quality of Abdala as a COVID-19 vaccine.
“We already announced the efficacy against symptomatic COVID, but there are other variables of the clinical study, which will also reveal efficacy against severe and asymptomatic forms of the disease, and we also want to evaluate what happened after the first and second doses,” explained the expert, according to which, as part of the trials carried out, “all the exudates of the volunteers that were PCR positive” have been isolated and “it is planned to carry out a study with the institutions in the country that have the capacity to do the isolation and the sequencing of the virus, as part of a scientific investigation that is of great interest.”
And with regard to the new strains, Dr. Ayala announced that “in our laboratories we have been obtaining recombinant proteins with the mutations of these variables, which we have been evaluating with the serums of individuals vaccinated with Abdala.” As a result, she said, “we have found that a recognition of these serums is maintained with these proteins, which is indicative that in the immune response that the vaccinated individual have there is an ability to recognize and, why not, dominate the new variable they are facing.”
What’s to come
Following the announcement of the efficacy of Soberana 02 and Abdala, the next step for both candidates to finally become vaccines is the approval of their use on a large scale by regulatory authorities. In this direction, Abdala’s file was already presented by the CIGB to the Center for the State Control of Medicines, Medical Equipment and Devices (CECMED), for authorization for its emergency use, while that of Soberana 02 will be delivered by the IFV next week, while work continues to calculate the efficacy of the scheme with a third dose of Soberana Plus.
In this regard, Dr. Eduardo Martínez, president of the BioCubaFarma group, commented to the press that, thanks to the positive results obtained in the calculation of the efficacy and the rest of the studied indicators, it is expected that said authorization could be granted “in the upcoming weeks” after the pertinent analysis of the Cuban regulatory entity. For his part, Dr. Yury Valdés, deputy director of the IFV, recalled that this authorization is not requested based on a single result and that the file to be submitted includes “all the information that has been worked on with the vaccine candidates, the results and accumulated clinical evidence, partial reports and other rigorous elements such as the inspections carried out of the production systems.”

Dr. Eduardo Martínez, president of the state group BioCubaFarma, in charge of the development of the Cuban COVID-19 vaccines. Photo: Otmaro Rodríguez.
Once the permit is granted, two important roads open. First, the mass vaccination in Cuba, which has already been carried out under the studies and health interventions underway — which have made it possible to administer until now more than 5.3 million doses of Cuban vaccine candidates and begin immunization of about 2.5 million people on the island — and secondly, the presentation of the results to journals and international organizations, which support and validate what would be officially the first COVID-19 vaccines developed in Latin America.
Regarding vaccination, Dr. Rolando Pérez, director of science and research at BioCubaFarma, confirmed that this process is in charge of the Ministry of Public Health (MINSAP), although with the participation of scientists in charge of vaccines, and assured that “the fundamental principle that governs the immunization strategy is the epidemiological situation of the country.”
“It is, above all, about intervening in the populations and territories at risk, in the regions where we have the most epidemiological problems. These will be the ones that will be prioritized, so that we can cover the places most affected by the disease, with the aim of reducing the incidence. We cannot say now which territory will be first and which will be later, because they are analyzes that are carried out continuously, according to the availability of vaccines and the epidemiological scenario, and with these elements it is decided how to move forward,” explained the executive.
Regarding the presentation of the results to global entities and their possible validation by them, Dr. Pérez assured that Cuban specialists and health authorities have held systematic exchanges with both the WHO and the Pan American Health Organization (PAHO), to update them on the progress of the Cuban vaccine candidates, and that “once the emergency authorization is obtained in Cuba, we can really plan to enter a certification process.”
“It has been a permanent dialogue,” said the specialist, who announced that after the announcement of the efficacy percentages of Soberana 02 and Abdala, the Cuban side established communication with PAHO and “we have coordinated a next meeting, to facilitate the exchange of information with experts in Washington and also from the WHO.”
A third path that is opening, or rather is being reinforced, is the export of Cuban COVID-19 vaccines, based on previous agreements and conversations with several countries and the growing interest of others after the release of the efficacy data. Nations such as Venezuela, Argentina, Mexico and Vietnam are among those that have already contacted Cuba, while others from Latin America and also from Africa and the Caribbean could join the list. Meanwhile, Iran has been carrying out its own clinical trial of Soberana 02 — renamed Pasteur there — and is preparing to authorize its emergency use in the coming days.
Cuba envió a Venezuela 30 mil dosis de su candidato vacunal Abdala
But beyond this, Cuban scientists do not stop and research is ongoing — to obtain more data from the studies already carried out and also to advance new trials in groups such as convalescents and in pediatric ages, and with other vaccine candidates —, as well as the productive escalation necessary to immunize the Cuban population at the expected rate and to be able to commercialize the vaccines internationally. In this sense, the greatest challenge comes from the difficulties in obtaining certain supplies and materials necessary for the manufacture of Cuban immunogens, due to their shortage and competition for them generated by the pandemic, and the harmful effects of the U.S. embargo/blockade.
Even so, the island’s specialists claim to be working on technological solutions to face these difficulties and to have a better knowledge of the production process for the sake of successful escalation, which is why they maintain the objective of manufacturing “millions of doses” this year. With this they hope to fulfill the purpose of vaccinating 70% of the Cuban population at the end of August and making the island the first country in the world to immunize its entire population with its own vaccines.









What was the placebo?