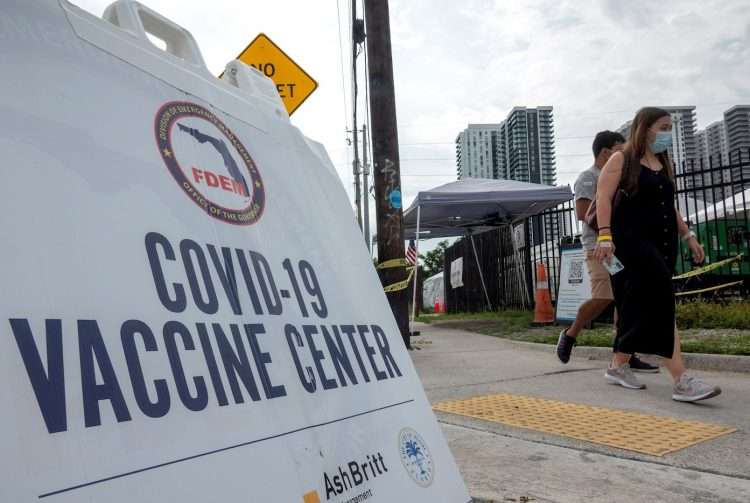
The United States will ask travelers arriving in the country as of November 8 to be immunized against COVID-19, even when a mix of doses of different types of vaccines have been administered, provided that these have been approved by the World Health Organization (WHO).
This was confirmed by the latest update of the U.S. Centers for Disease Control and Prevention (CDC) guidelines, which further confirms that immunization tests will only be accepted with those types of vaccines that have been approved by the WHO.
A spokeswoman for the U.S. Centers for Disease Control and Prevention (CDC) told the EFE agency this Saturday that people who have a combination of two (different) doses of a double dose vaccine that has been cleared by the FDA (U.S. Food and Drug Administration) or is on the WHO emergency use list will be considered vaccinated with the complete scheme.
The source added that although the CDC has not recommended mixing vaccine types in a primary series, it recognizes that this is increasingly common in other countries, therefore it should be accepted for the interpretation of vaccination tests.
The White House announced this Friday that on November 8 it will open its air and land borders to international travelers vaccinated with the complete scheme against COVID-19, including those from those countries that have been subject to entry restrictions for more than a year, like Spain or Brazil.
The latest CDC update in this regard, published on its website late Friday, emphasizes that, for entering the United States, accepted vaccines will include those authorized or approved by the FDA or that are on the list of WHO emergency use.
EEUU reabrirá sus fronteras terrestres y aéreas el próximo 8 de noviembre
So far, the WHO has licensed all three FDA-approved vaccines — those from Pfizer/BioNTech, Moderna and Janssen (from Johnson & Johnson) —; and has also listed two versions of the AstraZeneca vaccine, in addition to Sinopharm and Sinovac.
That list does not include vaccines like the Russian Sputnik V and the Chinese CanSino, which are being administered in countries like Mexico; this led this week to the Mexican president, Andrés Manuel López Obrador, to ask the WHO to accelerate the process to include these serums on its emergency list.
“The WHO has to act with rectitude, without ideological tendencies, with adherence to science,” said López Obrador, who considered it “common sense” to authorize drugs that “have helped and have not harmed.”
The list also does not include Cuban immunogens, which have already been exported to countries such as Vietnam and Venezuela and which Mexico has shown interest in acquiring, although the island has already begun the process for their validation by the WHO.
The White House also announced this week that it will open the land borders with Mexico and Canada, which have been closed to non-essential travel since March 2020, coinciding with the outbreak of the pandemic and still with Donald Trump as president.
The plan to open those land borders will consist of two phases. The first will come into force on November 8 and will require proof of vaccination for trips considered “non-essential,” such as tourism or visits.
The second will be in January 2022, in which proof of vaccination will be mandatory for all travelers, including truckers, who will thus have time to get vaccinated before that requirement takes effect.
The lifting of the restrictions also benefits international travelers who are vaccinated and flying from countries hitherto subject to travel restrictions due to the pandemic, a list that includes the 26 European states of the Schengen area, in addition to the United Kingdom, Ireland, Brazil, China, Iran, South Africa and India.
EFE/OnCuba