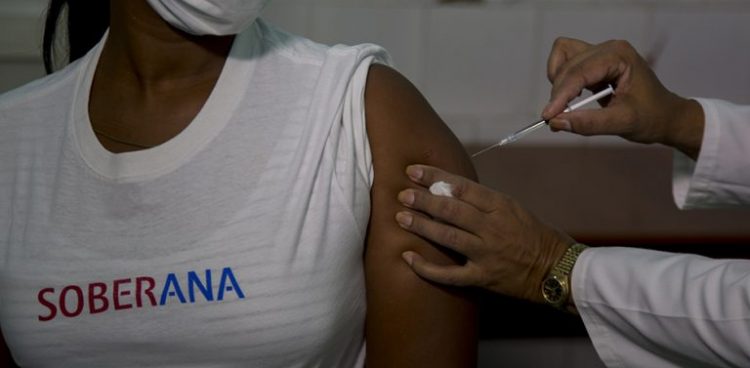
Cuba is carrying out a clinical trial in people convalescing from COVID-19, who had a minor clinical picture or were asymptomatic, the official media reported.
The study uses the Soberana 01 vaccine candidate, the first to receive authorization in Cuba for tests on human beings, and seeks to “stimulate protective levels of neutralizing antibodies against possible reinfection,” Prensa Latina (PL) agency reported.
The agency recalled that “globally there are cases of people infected with this coronavirus who were reinfected, even with much more acute and serious forms of the disease,” which is why the trial that is taking place on the island “also seeks to protect this risk group.”
The test began on January 9 at the Institute of Hematology and Immunology and involves 30 volunteers, aged 19 to 59, “who were in contact with the virus and currently have a negative PCR.” These people received a single dose of the vaccine on day 16 and “from that moment on, they are being closely monitored through doctor’s offices and direct contact with the researchers.”
#Soberana01: El ensayo comenzó el pasado día 9 de enero con reclutamiento de 30 voluntarios entre 19 y 59 años de edad, y continuó con la vacunación el 16 de enero. Ahora se realiza consulta de seguimiento, para evaluar la respuesta inmune.#CienciaCubanahttps://t.co/tT02RkVdN0
— Instituto Finlay de Vacunas (@FinlayInstituto) January 24, 2021
Dr. Rolando Ochoa, a specialist in Immunology at the Finlay Vaccine Institute, explained that the formulation of Soberana 01 used in the test “is simpler and safer, according to the characteristics of the population group under study.”
The expert argued that the vaccination was carried out in people convalescing from the disease, “who have low levels of neutralizing antibodies and, therefore, may be susceptible to reinfection,” Granma newspaper noted in this regard.
For his part, Dr. Arturo Chang, a specialist at the Institute of Hematology and Immunology, said that the study follows “the premise that these people were already vaccinated naturally (through contact with the virus), which is why this dose would be a boost to the individual’s immunity.”
The also principal investigator of the trial, which lasts for 28 days, explained that, “although what is conventional in phase I trials is that healthy and young people are chosen, the difference with this is that, when working with a population that already was in contact with the virus, it made it possible to include volunteers with some chronic pathology, but who are compensated.”
In addition, he specified, quoted by Granma, that “a second premise is related to the fact that the percentages of inhibition of antibodies against SARS-CoV-2 are relatively low, that is, that the person has not been immunized against the virus.” For this reason, he said “this trial is a novelty around the world, since the research has focused on the population sector that has not become ill.”
Chang stated that so far “there are no serious adverse events” and said that “if positive results are achieved in this phase, they will probably go on to a clinical trial with a larger number of subjects to evaluate the immunogenicity, efficacy and effectiveness of the product.”
Cuba has to date four COVID-19 vaccine candidates in the testing phase in humans: Soberana 01 and 02, both from the Finlay Institute, and Mambisa and Abdala, from the Center for Genetic Engineering and Biotechnology. Of these, Soberana 02 is the most advanced as it is already developing its phase II and Cuban scientists are confident that it can soon move on to phase III and be used in mass vaccination. To do this, they hope to produce 100 million doses this year.